Activated partial thromboplastin time assay
The activated partial thromboplastin time coagulation assay is one of the most frequently performed tests in hematology, and has a variety of uses in clinical practice. Accurate interpretation of the test depends on both clinical context (i.e. why the test was ordered) as well as an understanding of each laboratory’s normal reference range and assay sensitivity regarding detection of factor deficiencies, (unfractionated) heparin therapy and lupus anticoagulant.
by Dr Julianne Falconer and Dr Emmanuel J. Favaloro
Introduction
The activated partial thromboplastin time (APTT) assay is a commonly requested coagulation test, perhaps second only to the prothrombin time (PT)/international normalized ratio (INR), as used to monitor vitamin K antagonist (VKA) therapy such as warfarin. The APTT test assesses the intrinsic pathway of coagulation and has a variety of clinical uses; however, it is primarily used to screen for hemostasis issues, factor deficiencies, lupus anticoagulant (LA) or to monitor unfractionated heparin (UFH) therapy dosing. The test is sensitive to, but not specific for, detection of these abnormalities or influences. APTT prolongation may also be seen in liver disease, disseminated intravascular coagulation (DIC) and in the presence of factor inhibitors. Interpretation of an APTT result, be it normal or prolonged, is dependent on both the clinical context and the characteristics of the reagents and the assay as performed on particular instruments. The establishment of normal reference intervals (NRIs) and assessment of the assay in terms of its sensitivity to heparin, LA and clotting factors are important to provide accurate information for clinical interpretation [1].
Uses of the APTT assay
The APTT test is a global assay that measures the time to fibrin clot formation via the contact factor (‘intrinsic’) pathway (Fig. 1). The APTT test is usually performed on fully automated platforms, and involves activation of coagulation within the test (plasma) sample by the addition of specific reagents (containing phospholipids, contact factor activator and calcium chloride). The type of contact factor activator, and the type and concentration of phospholipid, used in the APTT reagent affects the sensitivity of the assay to, and thus its prolongation by, factor deficiencies, as well as to the presence of UFH and LA [1, 2].
The APTT is commonly used to monitor anticoagulation therapy using UFH (Table 1). It may also be prolonged, however, in the presence of VKAs including warfarin, as well as direct oral anticoagulants (DOACs) such as dabigatran (direct thrombin inhibitor) and rivaroxaban (anti-FXa inhibitor). The APTT is generally less sensitive to, but may still be slightly prolonged, by anticoagulation with low molecular weight heparin (LMWH) and with apixaban, another DOAC (anti-FXa inhibitor).
In the absence of anticoagulation therapy, an ‘isolated’ prolonged APTT may be used to determine a clinically important factor deficiency, for example as a screen for hemophilia A (FVIII deficiency), hemophilia B (FIX deficiency), or hemophilia C (FXI deficiency), or even von Willebrand Disease (VWD; which may be associated with loss of FVIII) [1]. An ‘isolated’ prolonged APTT, however, could instead be indicative of a clinically unimportant factor deficiency such as FXII or other contact factor deficiency. Other alternatives for an ‘isolated’ prolonged APTT include a factor inhibitor or LA. Despite causing prolongation of APTT in vitro, LA may be associated clinically with increased risk of thrombosis rather than bleeding. A prolonged APTT may be accompanied by a prolonged PT in the context of liver disease, DIC or fibrinogen (or other ‘common factor pathway’ deficiency/ies). Clinical context, therefore, must form the basis for accurate interpretation of APTT, be it either normal or prolonged, and together with other routine coagulation studies is essential to guide further investigations (Fig. 2).
A large number of commercial APTT reagents are now available, with wide variation in the type of contact factor activator and phospholipid source and concentration used. This will result in variation in sensitivity to all typical influences; thus also causing substantial variation in NRIs between APTT reagents, and requiring the establishment and verification of NRIs based on both the reagent and instrument in use. Unawareness of variation in APTT reagent sensitivity in context of clinical picture will lead to flawed clinical interpretation of results.
Establishing and verification of NRIs
A minimum of 20 normal individuals may be sufficient to establish a NRI for PT and APTT, according to guidance documents provided by the Clinical and Laboratory Standards Institute (CLSI) [3, 4]. However, a larger number of normal individuals is recommended to establish an initial NRI, following which a smaller sample of normal individuals may be used for future verification purposes [1].
As an example, Figure 3 shows an initial (historical) NRI estimation for APTT testing using a dataset of nearly 80 normal individuals. This included one outlier sample result (Fig. 3a), which was removed to produce the cleaner dataset used to produce the subsequent NRI. A statistical normality test was performed and showed the distribution to be near Gaussian, allowing parametric statistical assessment. For APTT testing, the NRI would aim to evaluate the 95 % confidence interval, approximating a mean
± 2 standard deviation (SD) assessment (Fig. 3b). Logarithmic transformation can instead be used to normalize test data when it is non-parametric and fits a log distribution (e.g. Fig. 3c).
If a NRI has been previously established by the laboratory or by the manufacturer of the APTT reagent using a specific reagent/instrument combination, the laboratory could use a process of transference to verify the ‘established’ NRI as fit for purpose. This may be done by establishing that a majority of samples in a small set of normal donors give values within the established NRI (e.g. >18 out of a set of 20 normal samples). Samples obtained from normal individuals or a dataset of normal patient test results may be used to assess a new lot of reagent to establish whether an existing NRI can be maintained when changing reagent lots.
Factor (deficiency) sensitivity
Factor sensitivity of an APTT assay (representing a specific reagent/instrument combination) can be assessed in a number of ways. One method involves serial dilution of either in-house or commercially derived normal plasma, into single-factor deficient plasma, in order to generate a series of aliquots with reducing factor levels. These samples are then tested by APTT and for factor level. The APTT reagent is regarded to be sensitive to the level of factor that correlates with the upper limit of the NRI.
A more accurate process, though particularly difficult to perform outside of a hemophilia centre, is to establish APTT values from true patients with various known factor levels [1, 2] (e.g. Fig. 4).
As a general guide, if the APTT is used for screening factor deficiencies, then the patient APTT value should be above the NRI when their factor level is below around 30–40 U/dL for FVIII, FIX, and FXI.
Sensitivity of APTT to UFH
Despite the changing landscape of anticoagulation therapy with the addition of direct anti-Xa inhibitors (rivaroxaban and apixaban) and a direct thrombin inhibitor (dabigatran) [5, 6], both LMWH and UFH continue to be frequently used in clinical practice. In turn, the APTT continues to be a generally preferred method of UFH monitoring over anti-FXa, given the wide availability and relative low cost of the assay. However, unlike the calibrated anti-FXa assay, APTT results are subject to variation between different instruments, be they be based on optical or mechanical clot detection methods [7], different APTT reagents (including variation between different lots of the same reagent type) and algorithms used on instruments for raw data processing. This poses a substantial problem with regards to historical recommendations to maintain patients on UFH between 1.5 and 2.5 times the ‘normal reference value’ (as based on limited evidence [8]). Therapeutic ranges should therefore be defined with specific reference to the instrument/reagent combination used locally [9].
One ‘spiking method’ involves testing samples containing known quantities of UFH diluted into normal pool plasma, as then tested by APTT and anti-FXa methods, allowing an estimation of the APTT therapeutic interval [1]. However, variations in certain components of patient plasma, as well as the non-physiologically processed nature of the UFH used, can impact on the interpretation of data obtained using this method. A better method involves ex vivo assessment of plasma obtained from patients on UFH therapy, with these tested for both APTT and anti-FXa, and then to establish a UFH therapeutic range for APTT that matches the therapeutic range for anti-FXa (e.g. 0.3–0.7 U/mL). It is important to recognize that individual response to UFH according to APTT is affected by many influences, including (but not limited to): antithrombin level; high or low levels of coagulation factors and proteins such as von Willebrand factor or proteins released from endothelial cells or platelets, competing with antithrombin for heparin binding; or increased FVIII levels in acute phase response; or reduction in FXII; or presence of LA (etc).
To obtain a cleaner data set to establish UFH therapeutic ranges, the following steps can be undertaken during sample collection and processing [1].
• Ensure baseline PT, APTT and INR testing prior to commencement of UFH are within their NRIs.
• Exclude underfilled samples, samples with visible hemolysis or likely platelet activation and release of heparin neutralizer platelet factor 4 (PF4).
• Exclude samples containing LMWH or other anticoagulants (e.g. VKAs, DOACs).
• Adhere to manufacturer guidelines with regards to the window from time of blood collection to testing.
• Double centrifuge samples when freezing them for batch testing (to remove residual platelets, which release PF4 and phospholipids on thawing).
• Accumulate data over a suitable time period to account for day-to-day test result variability.
• Aim for 30 or more data points.
• Appropriately dilute samples with anti-Xa activity above the test’s linearity limit.
• Remove data points reflecting ‘gross’ outliers.
LA sensitivity
The LA sensitivity of a particular APTT reagent can be assessed by comparing APTT tests of samples containing LA, for example by comparison of mean clotting times for each reagent.
Given that the APTT is a phospholipid-dependent assay, the test may be susceptible to prolongation in the presence of LA. However, differences in the phospholipid type and concentration between APTT reagents account for wide variation seen in the degree of prolongation of APTT, including due to LA. The LA sensitivity of the APTT reagent also has bearing on the use of APTT to monitor UFH and must inform the establishment of an algorithm to further investigate unexpectedly prolonged APTTs.
In one empirical method, initial testing using an LA sensitive method (e.g. dilute Russell viper venom time; dRVVT) is initially used to formulate a set of LA-positive samples of various ‘strengths’. Different APTT reagents can then be used to test the samples and the data for each sample can be plotted again the upper reference limit of the APTT for each reagent [1]. The ratio of clotting time of each LA-positive sample (of varying strengths) to the mean normal APTT derived from normal plasma samples is calculated. The median of these ratios allows different reagents to be ranked according to LA sensitivity. It can then become clear which APTT reagents are most (versus least) sensitive to LA. These can then be differentially selected according to the laboratory desire. For example, a laboratory may prefer to select an APTT reagent that is relatively LA ‘insensitive’, as combined with good factor VIII/IX/XI and UFH sensitivity if there is a desire to use a general purpose APTT screening reagent (i.e. hospital laboratory monitoring UFH, but wishing to avoid LA detection in asymptomatic patients). Alternatively, a laboratory may select an LA sensitive and an insensitive APTT reagent pair if they wish to assess for LA in symptomatic (thrombosis and/or pregnancy morbidity) patients.
Conclusion
Interpretation of a normal or a prolonged APTT must take into account both clinical context, including presence of anticoagulant therapy, as well as the methods and reagents used by the laboratory. The sensitivity of a particular APTT reagent to detect UFH therapy, LA and factor deficiencies has significant bearing on diagnostic assessment and therapy monitoring, and thus reflects essential knowledge for laboratory and clinical staff alike.
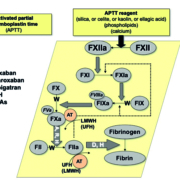
Figure 1. The activated partial thromboplastin time (APTT) assay measures the clot time to formation of fibrin via the contact factor pathway and is dependent on contact factors (FXII and above), and then FXI, FIX, FVIII, FX, FV, and FII. The APTT is also affected by vitamin K antagonists (VKAs; ‘W’), but more importantly is used to monitor unfractionated heparin (UFH; ‘H’) therapy and also to assess for potential hemophilia (FVIII, FIX or FXI deficiency). The APTT is also sensitive to the presence of other anticoagulants, including direct oral anticoagulants (DOACs) such as dabigatran (‘D’) and rivaroxaban (‘R’), and potentially also apixaban (‘A’) for some reagents. The APTT may also be utilized as part of a panel of tests to help assess for lupus anticoagulant (LA). (Modified from Favaloro EJ, et al. How to optimize activated partial thromboplastin time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost 2019; 45: 22–35 [1].)
Figure 2. An algorithm that provides one recommended approach for the follow-up of an abnormal APTT. Always exclude an anticoagulant effect first – there is no point investigating a prolonged APTT associated with anticoagulant use. Then consider the patient’s history, or the clinical reason for the test order, both of which assist in terms of follow-up approach. APTT, activated partial thromboplastin time; FBC/CBC, full blood count (UK/Australia)/complete blood count (USA); DIC, disseminated intravascular coagulation; DOAC, direct oral anticoagulant; EDTA, ethylenediaminetetraacetic acid; F, factor; LA, lupus anticoagulant; PT, prothrombin time. (Modified from Favaloro EJ, et al. How to optimize activated partial thromboplastin time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost 2019; 45: 22–35 [1].)
Table 1. The APTT test. A multipurpose and sensitive assay, but not specific for any individual parameter. List is not meant to be all inclusive.
DOACs, direct oral anticoagulants; VWD, von Willebrand disease.
*PT should also be prolonged if APTT is prolonged in the indicated setting.
(Modified from Favaloro EJ, et al. How to optimize activated partial thromboplastin time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost 2019; 45: 22–35 [1].)
Figure 3. Historical data from our laboratory to illustrate the process of deriving a normal reference interval (NRI) for the APTT, and using nearly 80 normal individual plasma samples. (a) APTT of all samples tested shown as a dot plot; one clear outlier shown as a red asterisk. (b) Data cleaned of outliers [i.e. in this case the single red asterisk sample in (a)]. (c) NRR estimate as mean ± 2 standard deviations (SDs) to provide approximate 95 % coverage. Bar graphs of parametric data processing and log transformed data processing shown. The NRI for this data set approximates 27–38 sec. (Modified from Favaloro EJ, et al. How to optimize activated partial thromboplastin time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost 2019; 45: 22–35 [1].)
Figure 4. Ex vivo heparin versus APTT evaluation. (a) Samples from all patients identified to be on heparin (as identified by our laboratory information system) and for which an APTT was performed at the time of evaluation are also tested for anti-FXa level. The APTT therapeutic range is that corresponding to a heparin level of 0.3–0.7 U/mL by anti-Xa. However, many data points in this figure do not reflect UFH alone. Some points may instead reflect low molecular weight heparin (e.g. likely to be the sample yielding an anti-Xa value close to 0.7 U/mL but with normal APTT) or alternatively UFH co-incident to FXII deficiency or LA, or else patients potentially transitioning from UFH to VKAs. These data points can be removed to yield a ‘cleaner’ data set, as shown in (b). (Modified from Favaloro EJ, et al. How to optimize activated partial thromboplastin time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost 2019; 45: 22–35 [1].)
Disclaimer: The views expressed in this paper are those of the authors, and are not necessarily those of NSW Health Pathology.
References
1. Favaloro EJ, Kershaw G, Mohammed S, Lippi G. How to optimize activated partial thromboplastin time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost 2019; 45: 22–35.
2. Kershaw G. Performance of activated partial thromboplastin time (APTT): determining reagent sensitivity to factor deficiencies, heparin, and lupus anticoagulants. Methods Mol Biol 2017; 1646: 75–83.
3. Defining, establishing, and verifying reference intervals in the clinical laboratory; proposed guideline—third edition. CLSI document C28–P3. Clinical and Laboratory Standards Institute (CLSI) 2008.
4. One-Stage Prothrombin time (PT) test and activated partial thromboplastin time (APTT) test; approved guideline—second edition. CLSI document H47-A2. CLSI 2008.
5. Favaloro EJ, McCaughan GJ, Mohammed S, Pasalic L. Anticoagulation therapy in Australia. Ann Blood 2018; 3: 48.
6. Lippi G, Mattiuzzi C, Adcock D, Favaloro EJ. Oral anticoagulants around the world: an updated state-of the art analysis. Ann Blood 2018; 3: 49.
7. Favaloro EJ, Lippi G. Recent advances in mainstream hemostasis diagnostics and coagulation testing. Semin Thromb Hemost. 2019; 45(3): 228–246.
8. Baluwala I, Favaloro EJ, Pasalic L. Therapeutic monitoring of unfractionated heparin – trials and tribulations. Expert Rev Hematol 2017; 10(7): 595–605.
9. Marlar RA, Clement B, Gausman J. Activated partial thromboplastin time monitoring of unfractionated heparin therapy: issues and recommendations. Semin Thromb Hemost 2017; 43(3): 253–260.
The authors
Julianne Falconer1 MBBS and Emmanuel J. Favaloro*1,2 PhD, FFSc (RCPA)
1Haematology, Institute of Clinical Pathology and Medical Research (ICPMR), NSW Health Pathology, Westmead Hospital, NSW, Australia.
2Sydney Centres for Thrombosis and Hemostasis, Westmead Hospital
*Corresponding author
E-mail: Emmanuel.Favaloro@health.nsw.gov.au