Clinical utility of PIVKA-II in the diagnosis of hepatocellular carcinoma
Primary liver cancer is the seventh most common cancer worldwide and the third most common cause of death from cancer [1]. Seventy-five to eighty-five percent of primary liver cancer cases are associated with HCC. The distinctive features of HCC include relatively large-sized tumours, vascular invasion, intra-hepatic metastasis, low differentiation, common recurrence and poor prognosis [2, 3]. In 70–90% of the cases, development of HCC requires a chronic liver disorder and cirrhosis as a background; these are caused mostly by chronic hepatitis C virus, hepatitis B virus, alcohol abuse, non-alcoholic steatohepatitis and less typically observed in inherited haemochromatosis, autoimmune hepatitis, antitrypsin deficiency, aflatoxin intoxication and also in some cases of oral contraception treatment [4, 5]. In addition, some chronic conditions, such as diabetes mellitus, cholelithiasis, obesity and hormone imbalance, are associated with HCC development [6]. The overall 5-year survival rate is believed to be less than 40%; however, a diagnosis at the early stages, followed by liver resection or transplantation, can improve this rate to 60–70% [7–9].
Taking into account the prevalence and mortality and also poor prognosis of HCC, it is apparent that highly sensitive techniques for diagnosis at the early stages are needed. The main diagnostic tool for HCC screening is radiologic imaging investigations such as ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI). With the development and introduction of contrast-enhanced ultrasound (CEUS) for analysis of intra-nodular vascularisation pattern, the sensitivity and specificity have been reported to be 90.9% and 100% for progressed HCC and 85.7% and 96.1% for early HCC, respectively [10]. The role of MRI and CT in producing reliable three-dimensional images is very important; however, the relationship between the radiographic and pathological tumour sizes is not yet well established. At this point application of tumour markers as supplementary analysis may provide useful information for making a diagnosis and monitoring of confirmed HCC [11, 12].
Protein induced by vitamin K absence/antagonist-II (PIVKA-II), also known as des-gamma carboxyprothrombin (DCP), is an abnormal form of prothrombin formed as a result of impaired or insufficient post-translational γ-carboxylation that occurs in the presence of vitamin K deficiency and leads to the loss of biological activity of the protein. Following synthesis in the liver, prothrombin, alongside the other hepatic vitamin K-dependent proteins undergoes transformation of specific glutamyl (GLU) residues into γ-carboxyl glutamyl (GLA) residues under the influence of vitamin K-dependent γ-glutamyl carboxylase in the presence of reduced vitamin K concentration (Fig. 1) [13]. Interestingly, carboxylation may not occur at all, which results in the formation of different variants of PIVKA-II with various degree of biological activity [14].
The role of PIVKA-II in HCC pathology is still not well established. It has been shown that PIVKA-II induces the malignant potential of HCC through stimulation of cell proliferation owing to a structural resemblance to hepatocyte growth factor [15–17]. Furthermore, PIVKA-II promotes angiogenesis in HCC resulting in local tissue invasion and metastases via stimulation of vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) [13, 18].
Methods and patient samples
The automated chemiluminescent microparticle immunoassay (ARCHITECT PIVKA-II 2P4 CMIA, Abbott) was validated and used for quantitation of PIVKA-II using the Abbott™ Architect iSystem 2000 analyser in the Human Nutristasis Unit at St Thomas’ Hospital, London, UK. Imprecision and recovery evaluations were performed in line with the appropriate standard operating procedures as part of the validation process. The CMIA is based on a two-step sandwich reaction of binding of anti-PIVKA-II antibodies and specific PIVKA-II epitopes with subsequent addition of chemiluminescent labels and registration of the relative light units as a quantitative representation of PIVKA-II concentration in the tested sample [1].
In order to exclude possible interference with anticoagulant therapeutic agents, high PIVKA-II results were tested for warfarin, as it is the most commonly used anticoagulant that interferes with the vitamin K cycle. Samples found to be positive for warfarin were disqualified from further analysis.
Eighty-seven samples from the Gassiott Gastroenterology Clinic (GGC, St. Thomas’ Hospital, London) and the Hepatocellular Carcinoma Clinic in the Institute of Liver Studies (King’s College Hospital, London) were analysed in three groups: high-risk patients with non-HCC pathology of the liver, high-risk patients currently undergoing HCC surveillance, and patients with diagnosed HCC (group A, B and C respectively). Group A (n=29) consisted of randomly selected patients at GGC with viral and non-viral cirrhosis, steatosis, fibrosis, hepatitis and benign lesions. Group B (n=24) represented high-risk patients with changes to the liver suggestive of possible HCC discovered in the course of US/MRI/CT investigations. Finally, group C (n=34) comprised of patients diagnosed with HCC at different stages; the diagnosis was established in the course of histological examination of liver biopsy samples.
All results for PIVKA-II concentrations in patient samples were statistically processed in IBM SPSS Statistics, Version 23. Tests of normality, association between different variables and receiver operating characteristic (ROC) curve were applied for the analysis.
Results and discussion
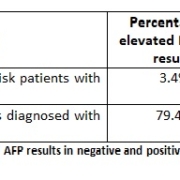
Using a cut-off of 49.4 mAU/mL, an elevated PIVKA-II concentration was found in just one patient from the negative control group, which represents 3.4% (Table 1). This patient was diagnosed with multiple cysts on the background of hepatitis; therefore, the result may be interpreted as both false positive (elevation of PIVKA-II due to non-malignant pathology) and true positive (in this case the patient would need to undergo more comprehensive screening).
In the positive group, PIVKA-II was elevated in 79.4% of the patients and demonstrated a broad scatter of values (19.06 mAU/mL for the lowest detected concentration and 340 485.5 mAU/mL for the highest detected concentration) owing to various sizes of the tumour masses at different stages of HCC and possibly existence of different PIVKA-II variants depending on the number of GLU residues involved in γ-carboxylation [19]. Normal PIVKA-II results in this group can be explained by the normalisation of PIVKA-II concentration after curative treatment, if performed [16].
Statistical processing of data showed no evidence of dependence of the results on age or gender (P>0.05 for all three groups). Area under the curve (AUC) in ROC analysis for PIVKA-II in the present research was 0.917 (CI 95% 0.847–0.986), which is suggestive of excellent clinical usefulness of PIVKA-II in HCC diagnosis (Fig. 2). AUC for alpha-fetoprotein (AFP) had slightly lower value (0.833 with CI 95% 0.722–0.945), which can still be classified as a fairly useful test (Fig. 3).
In this study the optimal cut-off value for PIVKA-II was identified by means of ROC and is 49.4 mAU/mL with sensitivity of 79.4% and specificity of 96.6%. Analysis of true and false-negative and -positive results revealed, that more than 83% of PIVKA-II results were truly reliable, whereas only 74.6% of AFP results demonstrated true diagnostic value (Table 2).
Unfortunately, sensitivity and specificity of AFP cannot accurately reflect its performance in the present study, as AFP results were available for only 17 patients from group A, which means that the study was possibly deprived of some potentially truly negative results. However, taking into account considerable difference between sensitivity and specificity rates for PIVKA-II and AFP (79.4 vs 96.6% and 70.6 vs 82.4% respectively), allows the conclusion that PIVKA-II displays slightly better clinical utility in HCC diagnosis. Similar results were reported in the previous studies [7, 20–24].
Limitations to the study
The major limitation to this research was the requirement to use anonymised samples, which prevented access to the full clinical history of the patients and impossibility to interpret the results in detail. Another limitation was the number of samples which could be considered to be insufficient to achieve aims of the project with adequate statistical power. A larger number of samples would have given the study more power and allowed a more precise ROC to be constructed and subsequently a more precise cut-off value to be identified.
Conclusion
In the present research PIVKA-II demonstrated high accuracy, sensitivity and specificity in HCC diagnosis. PIVKA-II has several advantages over AFP in terms of clinical utility for HCC diagnosis and prognosis: PIVKA-II is comparatively less frequently elevated in liver pathology [22], is more sensitive to small HCC tumours, correlates with HCC progression significantly better and has shorter half-life than AFP (40–72 hours against 5–7 days), which makes it more suitable for monitoring purposes [14]. Implementation of PIVKA-II as diagnostic test gathers pace in transplantation medicine, as this tumour marker, alongside Milan criteria has been used for recipient selection for living donor liver transplantation [16]. In addition, PIVKA-II concentrations can reflect the responsiveness of the liver to medical treatment (i.e. sorafenib), which cannot be achieved with AFP test. On the other hand, AFP is sensitive to radiological response following transarterial chemoembolisation, whereas PIVKA-II is not [12]. Also, PIVKA-II is affected by potentially interfering pharmacological agents (e.g. warfarin and certain antibiotics), it is dependent on vitamin K metabolism and can give false-positive results in non-HCC conditions which all has to be taken into account while interpreting the results.
Controversy over the best performance of tumour markers traces back to different assays used and various patient groups involved. Fortunately, AFP and PIVKA-II are independent of each other [16, 25]. Therefore, combination of PIVKA-II and AFP alongside AFP-L3, the fucosylated fraction of AFP, is suggested to be the best option for highly accurate laboratory diagnostic of HCC supplementary to imaging techniques. This multi-marker approach has been stated in the guidelines of The Japan Society of Hepatology and successfully used for diagnosis and management of HCC in Japan [26, 27].
Acknowledgement
ARCHITECT PIVKA-II 2P4 CMIA reagents and the graphics used in this article are courtesy of © Abbott Laboratories.
References
1. Kinukawa H, et al. characterization of an anti-PIVKA-II antibody and evaluation of a fully automated chemiluminescent immunoassay for PIVKA-II. Clin Biochem 2015; 48: 1120–1125.
2. Ha TY, et al. Expression pattern analysis of hepatocellular carcinoma tumour markers in viral hepatitis B and C patients undergoing liver transplantation and resection. Transplant Proc 2014; 46: 888–893.
3. Yano Y, et al. Clinical features of hepatitis C virus-related hepatocellular carcinoma and their association with α-fetoprotein and protein induced by vitamin K absence or antagonist-II. Liver Int 2006; 26: 789–795.
4. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–2576.
5. Aghemo A, Colombo M. Hepatocellular carcinoma in chronic hepatitis C: from bench to bedside. Semin Immunopathol 2012; 35: 111–120.
6. McMasters K, Vauthey J. Hepatocellular carcinoma: targeted therapy and multidisciplinary care. Springer 2011; Chapters 1–5, 8.
7. Ji J, et al. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepatocellular carcinoma in China: a large-scale, multicentre study. PLoS One 2016; 11: e0153227.
8. Huang TS, et al. Diagnostic performance of alpha-fetoprotein, lens culinaris agglutinin-reactive alpha-fetoprotein, des-gamma carboxyprothrombin, and glypican-3 for the detection of hepatocellular carcinoma: a systematic review and meta-analysis protocol. Syst Rev 2013; 2: 37.
9. Song PP, et al. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol 2016; 22: 262–274.
10. Giorgio A, et al. Characterization of dysplastic nodules, early hepatocellular carcinoma and progressed hepatocellular carcinoma in cirrhosis with contrast-enhanced ultrasound. Anticancer Res 2011; 31: 3977–3982.
11. Chen H, et al. CT and MRI in target delineation in primary hepatocellular carcinoma. Cancer Radiother 2013; 17: 750–754.
12. Park H, Park JY. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. BioMed Research International 2013; 2013: 310427.
13. Yue P, et al. Des-γ-carboxyl prothrombin induces matrix metalloproteinase activity in hepatocellular carcinoma cells by involving the ERK1/2 MAPK signalling pathway. Eur J Cancer 2011; 47: 1115–1124.
14. Zhang YS, et al. Des-γ-carboxy prothrombin (DCP) as a potential autologous growth factor for the development of hepatocellular carcinoma. Cell Physiol Biochem 2014; 34: 903–915.
15. Suzuki K, et al. Positioning of novel tumor marker NX-PVKA-R in the diagnosis of hepatocellular carcinoma in comparison with PIVKA-II. Dokkyo Journal of Medical Sciences 2013; 40: 163–168
16. Inagaki Y, et al. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin. Liver Int 2010; 31: 22–35.
17. Jinghe X, et al. Vitamin K and hepatocellular carcinoma: the basic and clinic. World J Clin Cases 2015; 3: 757–764.
18. Fujikawa T, et al. Significance of des-gamma-carboxyprothrombin production in hepatocellular carcinom. Acta Med Okayama 2009; 63: 299–304.
19. Zakhary NI, et al. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J Adv Res 2013; 4: 539–546.
20. Mathew S, et al. Biomarkers for virus-induced hepatocellular carcinoma (HCC). Infect Genet Evol 2014; 26: 327–339.
21. Lim TS, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol 2015; 51: 344–353.
22. Seo SI, et al. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 2015; 21: 3928–3935.
23. De J, et al. A systematic review of des-γ-carboxy prothrombin for the diagnosis of primary hepatocellular carcinoma. Medicine 2016; 95: e3448.
24. Ette AI, et al. Utility of serum des-gamma-carboxyprothrombin in the diagnosis of hepatocellular carcinoma among Nigerians, a case–control study. BMC Gastroenterol 2015; 15: 113.
25. Choi JY, et al. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol 2013; 19: 339–346.
26. Kudo M. Clinical practice guidelines for hepatocellular carcinoma differ between Japan, United States, and Europe. Liver Cancer 2015; 4: 85–95.
27. Kokudo M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015; 45: 123–127.
The authors
Volha Klimovich*1 MSc; Kieran Voong2 MSc; Roy Sherwood3 MSc, DPhil; Dominic J Harrington2 MSc, PhD
1Clinical Biochemistry, Viapath, St Thomas’ Hospital, London, UK
2Human Nutristasis Unit, Viapath, St Thomas’ Hospital, London, UK
3Viapath, King’s College Hospital, London, UK
*Corresponding author
E-mail: klimovichvolha@gmail.com