Aqueous humour biomarkers for retinoblastoma, a pediatric ocular malignancy
For decades, attempts to biopsy or obtain fluid from eyes with retinoblastoma had been contraindicated, however recent changes in the management of retinoblastoma have allowed for safe sampling of the aqueous humour (AH) during therapy. Use of the AH as a liquid biopsy enables tumour biomarker analysis in these eyes; this has potential to dramatically alter the management of this pediatric cancer.
by Dr Benjamin K. Ghiam, Dr Liya Xu and Dr Jesse L. Berry
Introduction
Retinoblastoma (Rb) is the most common intraocular cancer in children, comprising 4 % of all pediatric malignancies [1, 2]. This potentially fatal malignancy often goes undiagnosed until the tumour is advanced and has damaged intraocular structures. Survival rates for Rb are in excess of 90 % in developed countries, though a critical, and often challenging, focus of Rb therapy is globe and vision preservation [3]. Throughout decades of ocular medicine and surgery, any attempt to biopsy these tumours, or even obtain fluid from Rb eyes had been fervently contraindicated for risk of tumour seeding and dissemination. Thus, much of the diagnosis and management of Rb is dependent on information gathered by the ophthalmologist through careful eye examination, and without histopathologic evidence.
In 2012, Munier et al. described a safety-enhanced protocol for intravitreal chemotherapy injections in the eyes of patients with Rb; this protocol requires an initial paracentesis [4]. As described by the authors of the study, a volume of 0.1 ml of aqueous fluid is aspirated to induce transient hypotony before the intravitreal injection as a safety measure to prevent reflux to the injection site. This protocol for intravitreal injection of chemotherapy has now been widely adopted worldwide and the risk of extraocular spread is considered extremely low (zero reported cases with the safety-enhanced procedure) [5]. This demonstrated safety record paved the way for aqueous humour (AH) extraction in eyes with Rb undergoing active therapy.
AH is the clear intraocular fluid produced by the ciliary processes that fills the front part of the eye (anterior chamber). The AH functions to maintain intraocular pressure, provide nutrients to the cornea, and remove waste products. It has also been shown to be a rich source of information for intraocular disease, including Rb [6]. Researchers have long sought to evaluate AH for the presence of biomarkers which may correlate with features of intraocular disease and provide diagnostic and prognostic value. However, before 2017, any evaluation of the AH was only done on eyes after enucleation. Now that the AH can be safely extracted during therapy, we hypothesized that previous evaluations of AH biomarkers (post-enucleation) may now be clinically applicable for the diagnosis, prognosis and/or management of Rb. This article excerpts our recently published systematic review, titled “Aqueous Humor Biomarkers for Retinoblastoma, a review” in the journal Translational Vision Science and Technology [7].
Lactate dehydrogenase
Lactate dehydrogenase (LDH) is an enzyme found in nearly all cells that acts as a regulator of metabolism; it has been used clinically as a non-specific marker found within body fluids in various pathological conditions, including malignant tumours.
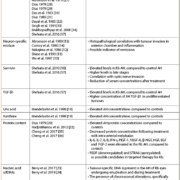
In the early 1970s, Dias et al. examined LDH levels in the AH from enucleated Rb eyes [8]. Early reports demonstrated a significant increase in the levels of LDH within the AH of enucleated eyes with Rb when compared to patients without Rb, such that levels >1000 U/L strongly support the diagnosis of Rb (Table 1). Multiple studies on LDH levels in the AH from enucleated eyes were done between the years 1971 and 2008 which found that LDH levels were significantly elevated compared to controls, and more elevated in advanced eyes with delayed diagnosis; however, these levels did not correlate with other clinical features or outcomes. Elevation in AH LDH have been described in patients with other ocular conditions, including primary open angle glaucoma and Coats’ disease. Although LDH was the first described marker of tumour activity in the AH, the lack of specificity and correlation with patient or tumour features limits its use clinically. Owing to this lack of correlation this research was previously abandoned.
Enolase/neuron-specific enolase
Neuron-specific enolase (NSE) is an isoenzyme of the glycolytic enzyme enolase; it is highly specific for neurons and peripheral neuroendocrine cells. Increased body fluid levels of NSE occur with malignant proliferation and thus have been of value in the diagnosis and characterization of neuroendocrine tumours, including small cell lung cancer and retinoblastoma [9].
Evaluation of the isoenzyme patterns of enolase in the AH of enucleated Rb eyes demonstrated that NSE levels were elevated in AH Rb, whereas enolase was not detectable in the AH from controls (Table 1) [10–12]. Elevated levels of NSE significantly correlated with inflammation and tumour invasion into the anterior chamber [13]. NSE levels did not correlate with histological tumour parameters (tumour necrosis, calcification, optic nerve/choroidal invasion) as well as clinicopathological parameters (sex, enucleation age, presentation age, family history, previous treatment, and metastatic disease). Moreover, NSE levels were found to be within the control range in children more than 5 years after active therapy [14]. This suggests that NSE may be used clinically to indicate remission status. Although obtaining serial AH NSE measurements may have a significant role in determining tumour status in Rb patients in the future, additional evidence is required to further substantiate the use of this tumour marker clinically.
Surviving and transforming growth factor beta-1
Survivin is a protein that inhibits apoptosis. It has garnered significant interest as a diagnostic and prognostic factor in human neoplasms, including Rb. Elevated survivin levels are found in many human neoplasms, and it is used as a prognostic factor in several human neoplasms, including lung and colorectal cancers [15, 16]
Survivin expression in the AH from enucleated eyes of children with Rb was found to be significantly elevated, when compared to patients with non-malignant ophthalmic disease, such as congenital cataracts and glaucoma [17, 18]. AH survivin levels correlated with tumour stage and histopathologic post laminar optic nerve involvement.
Transforming growth factor beta-1 (TGF-β1) expression in the AH of enucleated Rb eyes was associated with poor differentiation of the tumour [17]. The authors demonstrated high sensitivity and specificity of these AH proteins which makes them promising markers for Rb, particularly of more aggressive pathologic features.
Uric acid and xanthine
During cell turnover, nucleic acids and nucleotides are degraded into xanthine and uric acid. Elevated levels of serum uric acid have been associated with many malignancies, as well as after rapid destruction such as after treatment with chemotherapy or radiation.
Elevated concentrations of uric acid and xanthines were found in the AH of children with Rb compared with control eyes (Table 1) [19]. Elevated levels of xanthine and uric acid in AH may support the diagnosis of Rb in children suspected of having the disease, however further studies are necessary to establish optimal cut-offs, explore clinicopathological correlations, and compare Rb levels to lesions simulating Rb (Coats’ disease and persistent fetal vasculature).
Protein content
Normally, the AH is virtually protein-free to ensure a clear optical media between the cornea and the lens. An increase in globulin content and an albumin/globulin ratio < 1 has been found in enucleated eyes with Rb [20]. Concentrations of interleukin (IL)-6, IL-7, IL-8, interferon gamma (IFN-γ), placental growth factor 1 (PlGF-1), vascular endothelial growth factor A (VEGF-A), beta-nerve growth factor (β-NGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF-2) were significantly higher in the AH of patients with Rb than those in the control group [21]. Additionally, significantly decreased protein concentration was demonstrated in Rb eyes following treatment with selective intra-arterial chemotherapy (melphalan injection in the ophthalmic artery) that were subsequently enucleated after attempts at salvage, compared to primarily enucleated eyes [22].
Nucleic acids
Recent studies from Berry et al. demonstrated the presence of tumour-derived nucleic acids (DNA, RNA, miRNA) in the AH of Rb eyes [23]. Because of this, the authors suggest that the AH may be a rich source of tumour DNA and, thus, could be used as a liquid biopsy in children with Rb, without undergoing enucleation. A subsequent analysis by Berry et al. in 2018 showed that evaluation of the cell-free DNA (cfDNA) in the AH for chromosomal alterations has potential prognostic value as in indicator of aggressive disease [24]. Specifically, there was a significant increased odds of an eye failing therapy and requiring enucleation due to persistent and/or progressive cancer activity if gain of chromosome 6p was found in the AH cfDNA. Further research is required before this can be applied clinically, however this holds potential as a prognostic biomarker for Rb.
Conclusion
Despite significant investigation into tumour biomarkers for Rb spanning more than four decades, currently there are no active uses for the AH in a clinical setting. Diagnosis is made on the basis of examination and ancillary imaging findings without a biopsy, and molecular tumour markers are presently not used for diagnosis, prognosis, or to monitor therapeutic response. This is due in large part to the contraindication to biopsy in Rb; therefore, previously neither tumour nor AH or other ocular fluids were evaluated outside of specimens from enucleated eyes; clearly this limited the ability to correlate these markers with meaningful clinical outcomes. However, with recent advances in local therapy for Rb, paracentesis with extraction of the AH has now been shown to be safe in eyes being actively treated. This opens the door to for an AH liquid biopsy and thus there is renewed interest in these potential disease biomarkers.
Acknowledgement
This article excerpts our recently published systematic review, titled “Aqueous Humor Biomarkers for Retinoblastoma, a review” in the journal Translational Vision Science and Technology [7].
References
1. Shields, JA. Management and prognosis of retinoblastoma. In: Intraocular tumors: a text and atlas, pp377–391. WB Saunders 1992. ISBN 978-0721642680.
2. Shields JA, Shields CL. Intraocular tumors: an atlas and textbook, p574. Lippincott Williams & Wilkins 2008. ASIN B00XWR8WM6.
3. Pavan-Langston D. Manual of ocular diagnosis and therapy, p533. Lippincott Williams & Wilkins 2008. ISBN 978-0781765121.
4. Munier FL, Soliman S, Moulin AP, et al. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol 2012; 96(8): 1084–1087.
5. Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol 2013; 98(3): 292–297.
6. Macknight AD, McLaughlin CW, Peart D, et al. Formation of the aqueous humor. Clin Exp Pharmacol Physiol 2000; 27(1-2): 100–106.
7. Ghiam BK, Xu L, Berry JL. Aqueous humor markers in retinoblastoma, a review. Transl Vis Sci Technol 2019; 8(2): 13.
8. Dias PL, Shanmuganathan SS, Rajaratnam M. Lactic dehydrogenase activity of aqueous humour in retinoblastoma. Br J Ophthalmol 1971; 55(2): 130–132.
9. Kivelä T. Neuron-specific enolase in retinoblastoma. Acta Ophthalmol 2009; 64(1): 19–25.
10. Wu Z, Yang H, Pan S, et al. Electrophoretic determination of aqueous and serum neuron-specific enolase in the diagnosis of retinoblastoma. Yan Ke Xue Bao 1997; 13(1): 12–16.
11. Shine BS, Hungerford J, Vaghela B, et al. Electrophoretic assessment of aqueous and serum neurone-specific enolase in retinoblastoma and ocular malignant melanoma. Br J Ophthalmol 1990; 74(7): 427–430.
12. Nakajima T, Kato K, Kaneko A, et al. High concentrations of enolase, alpha- and gamma-subunits, in the aqueous humor in cases of retinoblastoma. Am J Ophthalmol 1986; 101(1): 102–106.
13. Abramson DH, Greenfield DS, Ellsworth RM, et al. Neuron-specific enolase and retinoblastoma. Clinicopathologic correlations. Retina 1989; 9(2): 148–152.
14. Comoy E, Roussat B, Henry I, et al. Neuron-specific enolase in the aqueous humor. Its significance in the differential diagnosis of retinoblastoma Ophtalmologie 1990; 4(3): 233–235 [in French].
15. Andersen MH, Svane IM, Becker JC, et al. The universal character of the tumor-associated antigen survivin. Clin Cancer Res 2007; 13(20): 5991–5994.
16. Rohayem J, Diestelkoetter P, Weigle B, et al. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res 2000; 60(7): 1815–1817.
17. Shehata HH, Abou Ghalia AH, Elsayed EK, et al. Clinical significance of high levels of survivin and transforming growth factor beta-1 proteins in aqueous humor and serum of retinoblastoma patients. J AAPOS 2016; 20(5): 444.e1–444.e9.
18. Shehata HH, Abou Ghalia AH, Elsayed EK, Z et al. Detection of survivin protein in aqueous humor and serum of retinoblastoma patients and its clinical significance. Clin Biochem 2010; 43(4-5): 362–366.
19. Mendelsohn ME, Abramson DH, Senft S, et al. Uric acid in the aqueous humor and tears of retinoblastoma patients. J AAPOS 1998; 2(6): 369–371.
20. Dias PL. Postinflammatory and malignant protein patterns in aqueous humour. Br J Ophthalmol 1979; 63(3): 161–164.
21. Cheng Y, Zheng S, Pan C-T, et al. Analysis of aqueous humor concentrations of cytokines in retinoblastoma. PLoS One 2017; 12(5): e0177337.
22. Hadjistilianou T, Giglioni S, Micheli L, et al. Analysis of aqueous humour proteins in patients with retinoblastoma. Clin Experiment Ophthalmol 2012; 40(1): e8–15.
23. Berry JL, Xu L, Murphree AL, et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol 2017; 135(11): 1221–1230.
24. Berry JL, Xu L, Kooi I, et al. Genomic analysis of aqueous humor cell-free dna in retinoblastoma predicts eye salvage: the surrogate tumor biopsy for retinoblastoma. Mol Cancer Res 2018; 16: 1701–1712.
25. Kabak J, Romano PE. Aqueous humour lactic dehydrogenase isoenzymes in retinoblastoma. Br J Ophthalmol 1975; 59(5): 268–269.
26. Piro PA Jr, Abramson DH, Ellsworth RM, et al. Aqueous humor lactate dehydrogenase in retinoblastoma patients. Clinicopathologic correlations. Arch Ophthalmol 1978; 96(10): 1823–1825.
27. Abramson DH, Piro PA, Ellsworth RM, et al. Lactate dehydrogenase levels and isozyme patterns. Measurements in the aqueous humor and serum of retinoblastoma patients. Arch Ophthalmol 1979; 97(5): 870–871.
28. Dias PL. Correlation of aqueous humour lactic acid dehydrogenase activity with intraocular pathology. Br J Ophthalmol 1979; 63(8): 574–577.
29. Dias PL. Prognostic significance of aqueous humour lactic dehydrogenase activity. Br J Ophthalmol 1979; 63(8): 571–573.
30. Das A, Roy IS, Maitra TK. Lactate dehydrogenase level and protein pattern in the aqueous humour of patients with retinoblastoma. Can J Ophthalmol 1983; 18(7): 337–339.
31. Dias PL. Electrolyte imbalances in the aqueous humour in retinoblastoma. Br J Ophthalmol 1985; 69(6): 462–463.
32. Dayal Y, Goyal JL, Jaffery NF, et al. Lactate dehydrogenase levels in aqueous humor and serum in retinoblastoma. Jpn J Ophthalmol 1985; 29(4): 417–422.
33. Singh R, Kaurya OP, Shukla PK, et al. Lactate dehydrogenase (LDH) isoenzymes patterns in ocular tumours. Indian J Ophthalmol 1991; 39(2): 44–47.
34. Mukhopadhyay S, Ghosh S, Biswas PN, et al. A cross-sectional study on aqueous humour lactate dehydrogenase level in retinoblastoma. J Indian Med Assoc 2008; 106(2): 99–100.
35. Cheng Y, Meng Q, Huang L, et al. iTRAQ-based quantitative proteomic analysis and bioinformatics study of proteins in retinoblastoma. Oncol Lett 2017; 14(6): 8084–8091.
36. Cheng Y, Zheng S, Pan C-T, et al. Analysis of aqueous humor concentrations of cytokines in retinoblastoma. PLoS One 2017; 12(5): e0177337.
The authors
Benjamin K. Ghiam1 MD, Liya Xu2 PhD, Jesse L. Berry, MD*3,4 MD
1Oakland University, William Beaumont School of Medicine, Rochester, MI, USA
2Department of Biological Sciences, Dornsife College of Letters, Arts, and Sciences, University of Southern California, Los Angeles, CA, USA
3The Vision Center at Children’s Hospital Los Angeles, Los Angeles, CA, USA
4USC Roski Eye Institute, Keck School of Medicine of USC, University of Southern California (USC), Los Angeles, CA, USA
*Corresponding author
E-mail: Jesse.Berry@med.usc.edu