Biomarkers for the diagnosis of sepsis
Sepsis is a medical emergency that needs rapid identification and treatment to create the best possible outcomes. However, in the early stages it can be very difficult to distinguish sepsis from uncomplicated infection. This article summarizes recent developments in sepsis nomenclature and definitions as well as providing an insight into the role that biomarkers might play in diagnosis and prognosis.
Background
Sepsis is a life-threatening condition associated with high morbidity and mortality, with the risk of death ranging from 30% to 80% depending on the severity of the disease. The World Health Organization estimates that more than 30 million people are affected by sepsis worldwide every year [1], although for reasons discussed by Candel et al., the actual epidemiology of sepsis is difficult to ascertain [2]. In the UK and USA it is thought that sepsis is the cause of around 37 000 and nearly 270 000 deaths per year, respectively [3, 4]. Outcomes of sepsis are better if it is detected and treated early, but despite the large numbers of people affected by it, public awareness of it is still low. In recent years, awareness campaigns have been launched and this year several popular TV and radio programmes in the UK have featured sepsis storylines (Call the Midwife, Coronation Street and The Archers).
Definitions
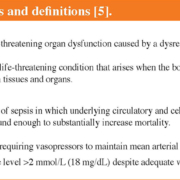
The difficulties experienced in studying the epidemiology of sepsis are likely to reflect the problems of characterization and diagnosis of the disease, which is in turn a reflection of the complex nature of the condition. Original definitions of sepsis date back to 1991, with the idea that sepsis was caused by systemic inflammatory response syndrome (SIRS) in resulting from infection. In 2001 the definitions were re-examined but left largely unchanged. In 2016, a task force re-evaluated and updated definitions of sepsis and septic shock (Box 1), taking into account improved understanding of the pathobiology of sepsis, which is now recognized to involve early activation of both pro- and anti-inflammatory responses, along with major modifications in non-immunologic pathways such as cardiovascular, neuronal, autonomic, hormonal, bioenergetic, metabolic, and coagulation [5]. A lay definition of sepsis published in 2011 [6] was also accepted by the 2016 task force (Box 1). The definitions created in 1991, 2001 and 2016 have been designated Sepsis-1, Sepsis-2 and Sepsis-3, respectively, to indicate the need for ongoing refinement.
Diagnosis of sepsis
Early diagnosis and treatment of sepsis is associated with improved outcomes, but the difficulty lies in distinguishing sepsis from uncomplicated infection. Identification of patients with sepsis is largely achieved through the use of the Sequential (or Sepsis-Related) Organ Failure Assessment (SOFA) score (Table 1) in the hospital setting or the quick SOFA (qSOFA) score (See Figure 1 “Operationalization of Clinical Criteria Identifying Patients With Sepsis and Septic Shock” in Singer et al. [5]). Commencement of treatment should occur within the first hour of admission and should not be delayed by waiting for results from the lab, as the SOFA score can be applied retrospectively. Management of sepsis also requires (amongst other things) that blood samples are taken before broad spectrum antibiotics are administered and that once the pathogen has been identified antibiotic usage can be refined to aid antimicrobial stewardship (See the Surviving Sepsis Campaign [7] and NICE guidelines [8] for full details of early sepsis management). Sepsis is most commonly caused by bacterial infection, but can also be due to fungal, viral or parasitic infection. However, identification of the pathogen and its antibiotic susceptibility and/or resistance by classic culture techniques is slow and molecular- and proteomic-based approaches, such as matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) spectroscopy, may improve turnaround times [9].
Biomarkers
The difficulty of distinguishing sepsis from uncomplicated infection has long driven the search for suitable biomarkers to aid sepsis diagnosis. An ideal biomarker would be able to distinguish sepsis from non-infectious causes of critical illness, having a fast and specific increase in sepsis and a rapid decrease after effective therapy. A number of potential biomarkers have been identified, although none are specific enough to be used alone.
Procalcitonin and C-reactive protein
The most-studied biomarkers are procalcitonin and C-reactive protein (CRP). CRP is an acute-phase protein that is secreted from the liver in the response to inflammatory processes and is therefore sensitive but not specific for sepsis. Procalcitonin, again is produced in response to inflammation and infection, and is so far the only biomarker to be used clinically, as it differentiates better than CRP between infectious and non-infectious causes of critical illness. A meta-analysis found that procalcitonin had a mean sensitivity and specificity of around 70% and an area under receiver operator characteristic curve of less than 0.80 [10]. However as levels of procalcitonin are known to be raised after surgery, trauma and viral infection, the Surviving Sepsis Campaign concluded that procalcitonin levels are not adequate to distinguish sepsis from other causes of inflammation [11], although it may be useful for indicating when treatment with antibiotics can end [12].
Interleukin 6 (IL-6)
IL-6 was initially a biomarker of interest for rapid sepsis diagnosis as it has a fast kinetic profile – the concentration increases within 2 hours of onset of sepsis and decreases within 6 hours. However, the results from studies have been mixed, with some suggesting that it was able to discriminate between sepsis and non-infectious illness, whereas others found that procalcitonin was better, hence it has not been added to current guidelines [11].
Promising biomarkers
A number of other biomarkers have been identified that show promise include soluble urokinase-type plasminogen activator receptor, presepsin and proadrenomedullin [2, 13]. Additionally, recently, reduced serum levels of fetuin-A (a major hepatokine) were found to be independently associated with predicting progression to septic shock and higher rates of mortality [14].
Biomarker panels
Even today, no single biomarker has the diagnostic strength to identify patients suffering from sepsis and it is likely that assessing panels of biomarkers will increase the sensitivity and accuracy of diagnosis of sepsis, compared to any individual biomarker (for example, see the study by Kofoed et al. [15]). More recently, the power of mass spectrometry and “-omics studies” is being investigated with some promise, although still suffering from limitations [13].
References
1. Sepsis. World Health Organization 2018; http://www.who.int/news-room/fact-sheets/detail/sepsis.
2. Candel FJ, et al. Current aspects in sepsis approach. Turning things around. Rev Esp Quimioter 2018; 31(4): 298–315.
3. Improving outcomes for patients with sepsis: a cross-system action plan. NHS England 2015; https://www.england.nhs.uk/wp-content/uploads/2015/08/Sepsis-Action-Plan-23.12.15-v1.pdf.
4. Sepsis. Centers for Disease Control and Prevention 2018; https://www.cdc.gov/sepsis/datareports/index.html.
5. Singer M, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8): 801–810.
6. Czura CJ. Merinoff symposium 2010: Sepsis – speaking with one voice. Mol Med 2011; 17(1-2): 2–3.
7. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Surviving Sepsis Campaign 2016; http://www.survivingsepsis.org/Guidelines/Pages/default.aspx.
8. Sepsis: recognition, diagnosis and early management; NICE guideline [NG51]. National Institutes for Health and Care Excellence 2017; https://www.nice.org.uk/guidance/NG51/chapter/Recommendations#identifying-people-with-suspected-sepsis.
9. Ward KM, Harris R. Sepsis: earlier organism identification using MALDI-TOF. Clin Lab Int 2015; Nov: 14–18.
10. Wacker C, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13: 426–435.
11. Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41(2): 580–637.
12. Sager R, et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med 2017; 15: 15.
13. Ludwig KR, Hummon AB. Mass spectrometry for the discovery of biomarkers of sepsis. Mol Biosyst 2017; 13(4): 648–664.
14. Karampela. Karampela I, Kandri E, Antonakos G, Vogiatzakis E, Christodoulatos GS, Nikolaidou A, Dimopoulos G, Armaganidis A, Dalamaga M. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: A prospective study. J Crit Care 2017; 41: 78–85.
15. Kofoed K, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care 2007; 11(2): R38.