Blood-based peripheral biomarkers for dementia: are we any closer to their use in the clinical setting?
Dementia is one of the leading causes of disability in old age and places a huge burden on society. The growing prevalence of dementia calls for accurate, more accessible biomarkers to facilitate clinical diagnosis and prognosis. Peripheral mediums, such as blood and blood derivates (i.e. plasma and platelets), are currently being investigated for their potential as biomarkers of dementia subtypes. There is a lack of reproducibility in dementia biomarker studies, likely because of unaccounted factors such as age, ethnicity and gender, which is stalling their translation from research to the clinical setting. However, several blood-based biomarkers have been consistently reported from plasma and blood cells, including amyloid and tau protein, clusterin and immunoglobulins, as well as α-synuclein. This review highlights the need for further validation of the current blood-based dementia biomarkers for their routine clinical use.
by Oluwatomi E. S. Akingbade and Prof. Elizabeta B. Mukaetova-Ladinska
Introduction
The increasing incidence of neurodegenerative diseases, such as dementia, legitimizes the search for readily accessible biological markers. At present, there are 50 million people worldwide living with dementia, with 10 million new cases reported each year [1]. Dementia care costs are as high as £26 billion a year [1], with the likelihood of increasing further owing to higher numbers of people living with dementia. The increasing incidence of dementia calls for more efficient diagnosis. Currently, dementia diagnosis relies on extensive clinical assessments, facilitated by invasive (i.e. lumbar puncture) and technical (i.e. MRI) testing, all costly and inefficient in keeping up with the growing number of dementia patients.
The definitive diagnosis of dementia is done neuropathologically, and besides the clinical evidence of dementia, is based on the characteristic hallmarks of plaques and tangles (for Alzheimer’s disease (AD), the most common form of dementia [2]), Lewy bodies (for dementia with Lewy bodies (DLB)), and vascular changes (for vascular dementia). Some of the molecular substrates of the characteristic neuropathological dementia features have been taken forward both in neuroradiological (i.e. β-amyloid radiotracers) and biochemical assessments [i.e. amyloid-β (Aβ42), total tau and phosphorylated tau181 measurements in the cerebrospinal fluid (CSF)] [3]. However, their costs, limited access and invasive approach, as well as involvement in secondary inflammatory processes in dementia [4], are restricting their wider clinical utility. Thus, there is a need for the development of less invasive and more cost-effective peripheral biomarkers to facilitate the clinical diagnosis of dementia.
Unlike CSF, blood and blood derivates (platelets and plasma) are easily accessible in the clinical setting and the potential of using them to corroborate dementia diagnosis will likely lead to earlier, and more accurate dementia diagnoses. Although the blood–central nervous system barrier provides a physiological and physical barrier, changes in peripheral fluids and organs have been identified in people with dementia. Notably, erythrocyte [5] and platelet [6] physiology and function are largely effected in dementia. Proteins in peripheral organs are also being explored: i.e. α-synuclein, p53 protein, tau and amyloid in the skin, kidneys and liver; tau protein in the testes; Aβ1–42 and acetylcholinesterase in serous fluid, as well as amyloid and tau proteins in the gastrointestinal tract. Explorative studies continue to report that peripheral components are influenced in dementia disease. Reoccurring peripheral proteins of interest in the search for dementia biomarkers include α-synuclein, Immunoglobulin G (IgG), Aβ precursor protein (AβPP), clusterin (all found in both platelets and plasma) and myeloperoxidase (MPO, present in plasma only).
Clinical relevance of potential blood-based dementia biomarkers
Peripheral Aβ and AβPP
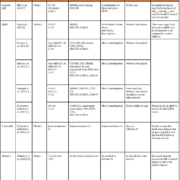
Aβ plaques in the brain are key pathological hallmarks of AD, and lower Aβ CSF level is a known marker in autopsy-confirmed AD subjects [22]. Aβ is also reported in the periphery, i.e. blood and skin. In a healthy population, plasma Aβ40, Aβ42 and Aβ40/42 ratio levels were significantly higher in older participants than in the younger ones [23]. In the Rotterdam Study Cohort [21], lower levels of plasma Aβ1–28 and Aβ40–42 were linked to higher risks of dementia in older age [7]. In platelets, the AβPP ratio [upper (130kDa) to lower (106–110kDa) immunoreactive bands] has been investigated (Table 1). Thus, there are differences in platelet AβPP ratios of those with cognitive deficits pertaining to dementia (i.e. very mild AD [10]; mild AD [10]; and AD [9–11]) and control patients. However, there are conflicting reports on the differences between the AβPP ratio in patients with varying severity of cognitive deficit with some studies [9,10]. Additionally, some studies have reported that increased AβPP levels in platelets correlated with higher Mini-Mental State Examination (MMSE) [11, 12] and Cambridge Cognition Examination (CAMCOG) scores [11]. Whether or not peripheral measurements of Aβ can be used as a pre-symptomatic marker of dementia (and/or the associated cognitive decline) remains uncertain. However, the studies mentioned in this review provide a consistent foundation for future studies to standardize measurement techniques that can be used in the clinical setting.
Peripheral α-synuclein
Studies have consistently reported the potential of α-synuclein as a biomarker of dementia – particularly in DLB as α-synuclein is reported to be a key constituent of Lewy bodies [24]. About 95 % of the α-synuclein in blood is predominantly found in erythrocytes, with the levels in plasma being extremely low. It is only recently that an assay was developed that is sensitive enough to detect the low levels of α-synuclein in plasma – reported in the range of 2.1–19.4 µg/L [25]. However, platelet α-synuclein levels in dementia have been reported to be similar among control and AD subjects [12] (Table 1). The utility of α-synuclein as a dementia biomarker is somewhat questionable, largely owing to its unknown physiological function in the human brain, as well as its involvement in Lewy body formation, which can be present in both normal aging as well as in a number of synucleinopathies, including Parkinson’s disease, multiple system atrophy, DLB, amyotrophic lateral sclerosis, etc, [26].
Blood clusterin
Increased plasma clusterin levels were reported to be indicative of both brain atrophy in AD patients [27] and an increased risk of dementia in older people [13]. Indeed. elevated levels of plasma clusterin have been reported as a risk factor for dementia [13, 28]. One study identified that lower MMSE cognitive scores in an AD population were associated with higher levels of plasma clusterin, whereas AD patients with higher cognitive scores had lower plasma clusterin levels [15]. Furthermore, longitudinal assessments identified that increased plasma clusterin concentrations are related to cognitive decline in mild cognitive impairment [28]. Interestingly, platelet levels of clusterin appear to be similar in both control and AD subjects [12, 16], but the ratio of platelet and plasma clusterin is positively correlated to specific neuropsychiatric inventory sub-categories, in particular agitation, apathy, motor aberrant behaviour and irritability seen in AD subjects [12] (Table 1).
Peripheral immunoglobulins
Increased IgG levels in plasma of AD subjects has been reported [17] with no link to disease progression. In an independent study, plasma IgG levels remained unaltered but increased in the platelet samples of the same AD subjects when compared to IgG levels in their control counterparts [12]. Inconsistent results found in IgG in plasma may be due to experimental constraints and, therefore, the potential of IgGs to form part of dementia diagnosis should not be ruled out. A recent study has reported that electrochemical techniques can be used to detect immunoglobulin in the plasma in the pg/mL range [29] – such sensitivity will likely aid in more consistent findings in plasma IgG studies in dementia.
Peripheral tau protein
CSF tau (and its ratio to Aβ) has been extensively investigated in relation to dementia. A recent literature review has addressed the limited clinical value of CSF tau biomarker studies [30], attesting to the need to address the uncertainty behind CSF tau as a suitable peripheral biomarker for dementia. More recently studies have focused on the presence of tau in the periphery. In plasma, high levels of tau were weakly associated with AD and were longitudinally associated with increased brain atrophy and poor cognition [18]. Platelet tau protein showed a more complex relationship than the reported plasma tau protein levels; studies have also reported a higher ratio between high to low molecular weight tau ratio in AD patients when compared to controls [19, 31] – with acknowledgement of no significant differences found between low or high molecular weight tau protein levels in the platelets of control and AD patients [19] (Table 1). Another study, reported a negative correlation between total tau and phosphorylated tau in the platelets of AD participants [20]. All in all, investigation into peripheral tau as a biomarker of dementia (and even specific dementia subtypes) is still in the early stages and requires further investigation before it can be considered for use in clinical diagnosis.
Summary and conclusions
Historically, the search for peripheral, blood-based dementia biomarkers has focused largely on the protein profiles of plasma and serum in dementia patients. This review, based on 13 dementia biomarker studies, has shown that proteins in the periphery are influenced by neurodegeneration in dementia. Namely, increased concentrations of Aβ40, Aβ42 and clusterin in plasma were all shown to be indicative of an increased risk of developing dementia in the elderly. Most recently, increased levels of plasma neurofilament light chain were reported to be closely related to amyloid processing in both mild cognitive impairment and AD, and to correlate with poor cognitive performance and AD-related brain atrophy and hypometabolism [32]. Changes in proteins in platelets were shown to coincide with cognitive decline in dementia: lower levels of AβPP and higher levels of clusterin were present in those with poorer performance in cognitive tests. Interestingly, increased plasma levels of tau protein were associated with brain atrophy while total and phosphorylated tau levels in platelets were negatively correlated. These findings provide encouraging evidence for the measurable presence of blood-based proteins that are closely linked to AD hallmarks that have the potential to be used in routine clinical setting not only for diagnosis, but also for severity and progression of the dementia process. The reproducibility and causes of possible heterogeneity, i.e. age, ethnicity, co-morbidity and genetics, that may influence protein expression at the periphery will need to be explored further before these biomarkers can be used routinely in the clinic, and their accuracy for distinct dementia subtypes will also need to be determined.
References
1. Dementia. World Health Organization 2017 (http://www.who.int/en/news-room/fact-sheets/detail/dementia).
2. Types of dementia. Alzheimer’s Research UK 2018 (https://www.alzheimersresearchuk.org/about-dementia/types-of-dementia/).
3. Andreasen N, Vanmechelen E, Vanderstichele H, Davidsson P, Blennow K. Cerebrospinal fluid levels of total-tau, phospho-tau and A beta 42 predicts development of Alzheimer’s disease in patients with mild cognitive impairment. Acta Neurol Scand Suppl 2003; 179: 47–51.
4. Veitinger M, Varga B, Guterres SB, Zellner M. Platelets, a reliable source for peripheral Alzheimer’s disease biomarkers? Acta Neuropathol Commun 2014; 2: 65.
5. Stevenson A, Lopez D, Khoo P, Kalaria RN, Mukaetova-Ladinska EB. Exploring erythrocytes as blood biomarkers for Alzheimer’s disease. J Alzheimers Dis 2017; 60(3): 845–857.
6. Akingbade OES, Gibson C, Kalaria RN, Mukaetova-Ladinska EB. Platelets: peripheral biomarkers of dementia? J Alzheimers Dis 2018; 63(4): 1235–1259.
7. Hilal S, Wolters FJ, Verbeek MM, Vanderstichele H, Kamran Ikram M, Stoops E, Ikram MA, Vernooij MW. Plasma amyloid-β levels, cerebral atrophy and risk of dementia: a population-based study. Alzheimers Res Ther 2018; 10: 63 (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6026500/).
8. Tang K, Hynan LS, Baskin F, Rosenberg RN. Platelet amyloid precursor protein processing: a bio-marker for Alzheimer’s disease. J Neurol Sci 2006; 240(1–2): 53–58.
9. Borroni B, Colciaghi F, Corsini P, Akkawi N, Rozzini L, Del Zotto E, et al. Early stages of probable Alzheimer disease are associated with changes in platelet amyloid precursor protein forms. Neurol Sci 2002; 23(5): 207–210.
10. Padovani A, Borroni B, Colciaghi F, Pettenati C, Cottini E, Agosti C, Talarico G, Cattabeni F, Lenzi GL, et al. Abnormalities in the pattern of platelet amyloid precursor protein forms in patients with mild cognitive impairment and Alzheimer disease. Arch Neurol 2002 Jan; 59(1): 71–75.
11. Zainaghi IA, Forlenza O V, Gattaz WF. Abnormal APP processing in platelets of patients with Alzheimer’s disease: correlations with membrane fluidity and cognitive decline. Psychopharmacology (Berl) 2007; 192(4): 547–553.
12. Mukaetova-Ladinska EB, Abdel-All Z, Dodds S, Andrade J, Alves da Silva J, Kalaria RN, O’Brien JT. Platelet immunoglobulin and amyloid precursor protein as potential peripheral biomarkers for Alzheimer’s disease: findings from a pilot study. Age Ageing 2012; 41(3): 408–412.
13. Weinstein G, Beiser AS, Preis SR, Courchesne P, Chouraki V, Levy D, Seshadri S. Plasma clusterin levels and risk of dementia, Alzheimer’s disease, and stroke. Alzheimer’s Dement 2016; 3: 103–109.
14. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975; 4(4): 518–525.
15. Hsu J-L, Lee W-J, Liao Y-C, Wang S-J, Fuh J-L. The clinical significance of plasma clusterin and Abeta in the longitudinal follow-up of patients with Alzheimer’s disease. Alzheimers Res Ther 2017 Nov; 9(1): 91.
16. Mukaetova-Ladinska EB, Abdel-All Z, Andrade J, Alves da Silva J, O’Brien JT, Kalaria RN. Plasma and platelet clusterin ratio is altered in Alzheimer’s disease patients with distinct neuropsychiatric symptoms: findings from a pilot study. Int J Geriatr Psychiatry 2015; 30(4): 368–375.
17. Bosman GJ, Van der Linden PA, Bartholomeus IG, De Man AJ, De Grip WJ, Van Kalmthout PJ. Erythrocyte aging in the demented elderly: a fluctuating process? Mech Ageing Dev 1998; 100(1): 53–58.
18. Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S2, Baker D2, Tan Hehir CA, et al. Plasma tau in Alzheimer disease. Neurology 2016; 87(17): 1827–1835.
19. Slachevsky A, Guzmán-Martínez L, Delgado C, Reyes P, Farías GA, Muñoz-Neira C, Bravo E, Farías M, Flores P, et al. Tau platelets correlate with regional brain atrophy in patients with Alzheimer’s disease. J Alzheimers Dis 2017; 55(4): 1595–1603.
20. Mukaetova-Ladinska EB, Abdell-All Z, Andrade J, da Silva JA, Boksha I, Burbaeva G, Kalaria RN, O’Brien JT. Platelet tau protein as a potential peripheral biomarker in Alzheimer’s disease: an explorative study. Curr Alzheimer Res 2018; 15(9): 800–808.
21. Hofman A, Brusselle GGO, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015; 30(8): 661–708.
22. Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009; 65(4): 403–413.
23. Kiko T, Nakagawa K, Satoh A, Tsuduki T, Furukawa K, Arai H, Miyazawa T. Amyloid beta levels in human red blood cells. PLoS One 2012; 7(11): e49620.
24. Koehler NKU, Stransky E, Shing M, Gaertner S, Meyer M, Schreitmuller B, Leyhe T, Laske C, Maetzler W, et al. Altered serum IgG levels to alpha-synuclein in dementia with Lewy bodies and Alzheimer’s disease. PLoS One 2013; 8(5): e64649.
25. Fjorback AW, Varming K, Jensen PH. Determination of alpha-synuclein concentration in human plasma using ELISA. Scand J Clin Lab Invest 2007; 67(4): 431–435.
26. Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature 1997; 388: 839 (http://dx.doi.org/10.1038/42166).
27. Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund LO, Westman E, Kinsey A, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry 2010 Jul; 67(7): 739–748.
28. Jongbloed W, van Dijk KD, Mulder SD, van de Berg WDJ, Blankenstein MA, van der Flier W, Veerhuis R. Clusterin levels in plasma predict cognitive decline and progression to Alzheimer’s disease. J Alzheimers Dis 2015; 46(4): 1103–1110.
29. Garyfallou G-Z, Ketebu O, Sahin S, Mukaetova-Ladinska EB, Catt M, Yu EH. Electrochemical detection of plasma immunoglobulin as a biomarker for Alzheimer’s disease. Sensors 2017; 17(11): doi: 10.3390/s17112464.
30. Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, Martin S. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane database Syst Rev 2017; 3: CD010803.
31. Neumann K, Farías G, Slachevsky A, Perez P, Maccioni RB. Human platelets tau: a potential peripheral marker for Alzheimer’s disease. J Alzheimers Dis 2011; 25(1): 103–109.
32. Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in Patients With Alzheimer disease. JAMA Neurol 2017; 74(5): 557–566.
The authors
Oluwatomi E. S. Akingbade1,2 and Elizabeta B. Mukaetova-Ladinska*2,3 MD, PhD, MRCPsych
1School of Life Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham, UK
2Department of Neuroscience, Psychology and Behaviour, University of Leicester, Leicester, UK
3Evington Centre, Leicester General Hospital, Leicester, UK
*Corresponding author
E-mail: eml12@le.ac.uk