Calprotectin use in primary care. Are testing criteria being followed?
In 2013, the National Institute for Health and Care Excellence (NICE) published guidelines recommending the use of fecal calprotectin as a screening test for differentiating between inflammatory bowel disease and irritable bowel syndrome. Since then, despite a relatively slow uptake with only a few laboratories offering the test, fecal calprotectin has garnered much interest with more hospitals incorporating it into their pathology services. Therefore, the question of how well primary care is using the service arises. This, along with a brief historical context of inflammatory bowel disease and its diagnosis together with implications of calprotectin testing are discussed here.
by Dr Benjamin Palmer, Dr Wisam Jafar and Steven McCann
Historical context
Inflammatory bowel disease (IBD) is a term used to describe two relapsing chronic gastrointestinal disorders: ulcerative colitis (UC) and Crohn’s disease (CD). Both cause considerable morbidity amongst young patients in whom they occur more frequently [1]. With an estimated prevalence of 2.5–3.0 million people affected in Europe the incidence of IBD is increasing [1]. Although the etiology of IBD is unknown several theories have been put forward the most important and frequently cited elements being: gut microbiota; genetic pre-disposition; environment and immune dysregulation [2]. The two main types of IBD seen in the clinical setting are UC and CD: definitive diagnosis and confirmation is provided by endoscopy and histology. However, in 5% of cases a definitive diagnosis cannot be established: in such cases patients are diagnosed with inflammatory bowel disease unclassified (IBDU) [3, 4].
The gastrointestinal tract is colonized by a large number of diverse bacteria living in symbiosis with their host (microbiota) [5]. Disruption to the diversity and quality of the gut microbiota (dysbiosis) is now implicated in the pathogenesis of both UC and CD [2, 5, 6]. Other important participants are cytokines: as their release causes intestinal inflammation they have been implicated in some of the clinical symptoms such as diarrhea [7–9].
Historically, the two types of IBD were differentiated by the different cytokines and mature CD4+ T helper (Th) cells found elevated in tissue biopsies. UC was characterized by elevated levels of Th2 cells and interleukin (IL)-4, -5, -10 and -13 cytokines, whereas CD was defined by high levels of Th1 cells and the interferon gamma (IFNγ) cytokine [3]. However, new emerging information suggests that it is not as cut and dried as this: emphasis has now shifted towards such pathways as IL-23/IL-17 activation of more pathogenic Th17 cells playing a more significant role in the pathophysiology of not just IBD but other inflammatory diseases [10].
Complicating the clinical scenario is the high prevalence (around 10−20% of the UK population) of an unrelated functional bowel disorder called irritable bowel syndrome (IBS): the true prevalence is thought to be higher as it is suspected that many people may not seek medical advice [11]. Although it does not cause serious morbidities, IBS manifests with symptoms similar to those of IBD, making diagnosis difficult. Unlike IBD, it is not associated with inflammation and, hence, this provides a means of making a differential diagnosis.
Until recently, the diagnosis of IBD was made by clinical evaluation and a combination of biochemical and mainly endoscopic investigations that often involved repeat consultations and testing [3]. This, taken together with an ageing population, an increased public awareness of bowel cancer and the introduction of national screening campaigns has led to increased service demands in endoscopy. The fact that fecal markers, such as calprotectin, are secreted by inflamed intestinal mucosa has led to the development of laboratory assays that can detect gastrointestinal inflammation. It was because of these challenges and recent developments that led the National Institute for Health and Care Excellence (NICE) to publish guidelines on the use of fecal calprotectin in differentiating IBD from IBS in adults provided cancer is not suspected and that local reporting guidelines along with appropriate quality assurance procedures are in place [12]. A cut-off of 50 μg/g is recommended in which all patients with a calprotectin level ≥50 μg/g may be suggestive of IBD and should be referred to Gastroenterology, whereas a calprotectin level <50 μg/g is unlikely to be caused by IBD and should, therefore, be managed with a presumptive diagnosis of IBS [12]. One key study used by NICE in recommending this threshold was carried out by Tibble et al. in which 602 outpatients with lower gastrointestinal tract symptom were included [13]. In this study the authors clearly demonstrated that the IBD group of patients differed significantly (P=0.001–<0.0001) from the IBS group of patients and that a cut-off level of 10 mg/L (equivalent to 50 μg/g) provided optimal diagnostic performance [13]. However, the cut-off level of any test is dependent on the method used and, therefore, each laboratory should establish their method-specific cut-off level [12].
IBD pathway and audit outcome
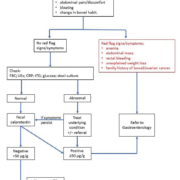
Following the recommendations of NICE, fecal calprotectin testing was incorporated into the pathology services at Stepping Hill hospital on 1 November 2014. The test is available for patients from primary care between 20 and 40 years of age who are presenting with abdominal pain or discomfort, bloating or altered bowel habits for 6 months or longer. Patients with red flag symptoms (anemia, abdominal mass, gastrointestinal infection, rectal bleeding, unexplained weight loss or a family history of ovarian or bowel cancer) should be referred directly to Gastroenterology (Fig. 1).
If the calprotectin test is negative the patient should be managed by primary care with a presumptive diagnosis of IBS; if the test is positive the patient should be referred to Gastroenterology. Then in 2016 a 1-year retrospective audit was carried out with the aim of determining how well primary care was adhering to the clinical pathway [14]. In order to achieve this, a questionnaire was designed and sent to all primary care surgeries in the catchment area for each patient who received calprotectin testing (n=587): the responses (n=217) to these questionnaires were then compared to the IBD pathway [14].
The outcome of this audit revealed that most areas of the IBD pathway were not being adhered to and, therefore, GP re-education and training was needed; a similar finding to another hospital [10]. The worst area of non-conformance was to ensure that patients had had signs/symptoms for at least 6 months: 69% of requests were not compliant [14]. Exclusion of gastrointestinal infection was second (63%), followed by ensuring age was 20–40 years (48%) [14]. Of the 216 questionnaires returned, 35% of patients had had red flag signs/symptoms at the time of the request [14]. Alarmingly, rectal bleeding was the most frequently encountered, followed by anemia, unexplained weight loss and a family history of bowel/ovarian cancer [14]. None of the patients had abdominal mass [14]. Conversely, high compliance was observed for the withdrawal of non-steroidal inflammatory drugs (NSAIDs) and antibiotics before testing [14]. Overall, only 24 requests (11%) were fully compliant for all criteria of the IBD clinical pathway [14].
Patient/clinician considerations
Unlike other fecal markers such as elastase, calprotectin is much less stable (up to 3 days at room temperature) and, therefore, it is important that patient samples are sent to the laboratory within 72 hours of collection [16]. It is still the case that samples are received by the laboratory with no date or time of collection and these are consequently rejected for analysis. Considering that calprotectin is elevated in gastrointestinal infection, in order for the test to be of clinical use, it is imperative that this is excluded.
The age restriction imposed on calprotectin testing is important and is based on the fact that IBS is more common in younger adults, that the prevalence of IBS decreases with increasing age and that new onset of symptoms after 50 years is uncommon [11, 17]. By focusing on this age group a large number of patients who do not require diagnostic testing will be excluded and, therefore, they will not add to the delay that current IBD sufferers face in receiving a colonoscopy.
Patients >40 years of age should be referred to Gastroenterology without delay, as the risk of developing colorectal cancer increases with age: colorectal cancer is the third most commonly diagnosed cancer in the UK and one of the major complications of IBD [18].
Since IBD is a relapsing disease the clinician should be aware that screening results from some patients with IBD may be negative, particularly if the disease is in a period of remittance and, therefore, the presentation of the patient, not the test result, should be the ultimate deciding factor over whether to refer. Finally, underpinning this is the need for local laboratories to determine their own method-specific cut-off values using evidence-based medicine. As with all screening tests the aim should be to optimize the test’s ability to exclude disease (IBS) so that fewer patients without disease are referred.
References
1. Trbojevic Akmacic I, Ventham NT, Theodoratou E, Vuckovic F, Kennedy NA, Krištic J, Nimmo ER, Kalla R, Drummond H, et al. Inflammatory bowel disease associates with proinflammatory potenial of the immunoglobulin G glycome. Inflamm Bowel Dis 2015; 21: 1237–1247.
2. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016; 186: 13–27.
3. Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol 2003; 16(4): 347–358.
4. Magro F, Giochetti P, Eliakim R, Ardissone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery and ileo-anal pouch disorders. J Crohns Colitis 2017; 11(6): 649–670.
5. Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory diseases. Inflamm Bowel Dis 2011; 17(1): 362–381.
6. Ni J, Wu GD, Alenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017; 14(10): 573–584.
7. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014; 14(5): 329–342.
8. Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol 2012; 18(41): 5848–5861.
9. Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1β attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem 2003; 278(49): 48794–48804.
10. Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 2006; 116(5): 1218–1222.
11. National Institute for Health and Care Excellence (NICE). Irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care. NICE Clinical Guideline 61, 2008 (https://www.nice.org.uk/guidance/cg61).
12. NICE. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. NICE Diagnostics Guidance 11, 2013 (https://www.nice.org.uk/guidance/dg11).
13. Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut 2000; 47: 506–513.
14. Palmer B, McCann S. A one year retrospective audit on calprotectin: how well is primary care adhering to the pathway for inflammatory bowel disease. Poster presented at Focus 2017, the Association of Clinical Biochemistry national annual meeting.
15. Turvill J. Evaluation of guidelines for the use of faecal calprotectin testing in primary care. NICE 2015. (https://www.nice.org.uk/sharedlearning/evaluation-of-guidelines-for-the-use-of-faecal-calprotectin-testing-in-primary-care).
16. Tøn H1, Brandsnes, Dale S, Holtlund J, Skuibina E, Schjønsby H, Johne B. Improved assay for faecal calprotectin. Clin Chim Acta 2000; 292: 41–54.
17. Halland M, Saito YA. Irritable syndrome: new and emerging treatments. BMJ 2015; 350: h1622.
18. Adelstein B, Macaskill P, Chan SF, Katelaris PH, Irwig L. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC gastroenterol 2011; 11: 65–74.
The authors
Benjamin Palmer*1 PhD, MRSC; Wisam Jafar2 MBChB, MRCP(gastro), MSc, MA, FRCP; Steven McCann2 MSc, FRCPath
1Betsi Cadwaladr Health Board, Glan Clwyd Hospital, Rhyl, Denbighshire, Wales, UK
2Stockport NHS Foundation Trust, Stockport, Cheshire, UK
*Corresponding author
E-mail: Ben.palmer@wales.nhs.uk