Chest pain management: high-sensitivity cardiac troponin supports rapid assessment of non-acute myocardial infarction patients
The introduction of cardiac troponin (cTn) assays has helped improve the triage of chest-pain patients. Evolution from relatively insensitive cTn assays to high-sensitivity assays has necessitated evolving testing approaches to optimize clinical utility. The latest generation (high-sensitivity cTn) support rapid diagnostic protocols aiding in the earlier discharge of a significant percentage of non-AMI patients, as well as aid a faster admission. The current 4th universal definition for AMI emphasizes that cTn can be elevated in many non-ischemic etiologies. To facilitate differentiation of an AMI, the guidelines define a rising or falling pattern of cTn assessed over time, in conjunction with other clinical information and risk assessment. The choice of clinical cutoffs and change values (delta) can be confounding, as cTn assays are not standardized. Testing algorithms such as a 0-3h protocol compared to rapid pathways supported by high-sensitivity assays (0 – 2h or 0 – 1h) also need to be taken into consideration. The use of the 99th percentile for cTn has been recommended since the first universal definition of AMI and continues to be currently recommended for 0-3h protocols, along with gender-specific cutoffs. For rapid diagnostic protocols, values well below the 99th percentile along with time-dependent deltas must be used. The sensitivity and precision offered by high-sensitivity assays is essential for rapid protocols in order to more accurately differentiate clinically significant change from assay imprecision. Rapid protocols identified for two recently available high sensitivity cTnI assays (High-Sensitivity Troponin I assays from Siemens Healthineers) will be reviewed, including performance in a 0 – 1h algorithm.
by Laurent Samson, PharmD and Katherine Soreng, PhD
Chest pain patients and AMI assessment
Patients with a chief complaint of “chest pain” suggestive of acute myocardial infarction (AMI) represent one of the most common ED presentations. As highly effective but time-dependent interventions for AMI exist, these patients are typically prioritized for assessment. While a diagnostic ECG can rapidly identify an ST-segment elevated myocardial infarction (STEMI), only a small percentage of patients have definitive ECG results. A larger percentage of patients with AMI lack clear ECG evidence but are experiencing a non-ST-segment elevated myocardial infarction (NSTEMI) and benefit from intervention. Both STEMI and NSTEMI fall into the category of the Acute Coronary Syndrome (ACS). Most chest pain patients have pain unrelated to ACS. The challenge in busy emergency departments (ED) is to rapidly identify STEMI and NSTEMI patients from those that can be safely discharged or evaluated for alternate etiologies. To aid diagnostic stratification, guidelines recommend serial biomarker testing with cardiac troponin I or T (cTnI, cTnT), with a rising/falling pattern indicative of evolving injury. High sensitivity troponin testing, in conjunction with other clinical findings and risk assessment, supports the differentiation of non-AMI patients from those experiencing cardiac ischemia.1
Evolving testing guidance is linked to cTn assay performance
In 2000, an expert consensus panel (the First Global MI Task Force) published a new AMI definition, which designated cardiac necrosis in the setting of myocardial ischemia be labeled as AMI. Recognizing the specificity of cTn, the authors adopted the 99th-percentile for cTn for a healthy reference population as the diagnostic threshold. An AMI was characterized by a rise and/or fall in values with at least one value above the decision level, along with a strong pre-test likelihood. This redefinition to a value just above that identified in a normal, healthy population dramatically increased AMI detection, and improved clinical confidence for exclusion. As cTn assays were (and are) not standardized (and cTnI is a different molecule than cTnT), the adoption of the 99th percentile vs. a “shared” numeric diagnostic cut-point was, and remains, necessary.
With adoption of the 99th percentile, low-end accuracy was crucial to better differentiate a true cTn elevation from assay imprecision. A precision criteria of <10% at the 99th percentile (upper reference limit or URL) was designated. While no assays available in 2000 could meet this definition for both sensitivity and precision, some manufacturers achieved approval of “guideline-compliant” or “contemporary sensitive” assays in subsequent years. As assay performance continued to improve and additional data to be published, recommendations evolved. In 2007, an update to the Universal Definition expanded the MI definition into five MI subcategories, each associated cTn values. The 99th percentile threshold continued to be recommended for Types 1 and 2 MI (typically occurring in patients presenting in the ED with chest pain) while multiples of the URL were designated for MI types 4 and 5.
The need for a changing pattern with at least one result above the diagnostic threshold in the setting of suspected myocardial ischemia was emphasized in the guidance. A changing pattern is essential to improve diagnosis of an AMI from chronic elevations associated with structural heart disease or alternate etiologies of cardiac damage. To assess change, cTn testing recommendations were 0 and 6-9 hours (with additional testing if AMI suspicion persisted).
In 2011, the ESC guidelines for the management of NSTEMI patients were published. The Expert Panel recognized the increased availability and improved performance for sensitive assays, and the development of high-sensitive cTn. Given the ability of high sensitivity assays to detect low levels of cTn with good precision, suggested testing intervals were shortened to 0 and 3-6 hours. In 2012, the updated 3rd Universal Definition was published, and recommended a 3h vs. 6h delta change if using a hs cTn. Similar guidance for a 0-3h protocol was published in the American guidelines in 2014 for the management of NSTE-ACS patients. Also, in 2014, the IFCC task force on cardiac biomarkers defined the high sensitivity troponin criteria and introduced the use of whole numbers with cTn (units of ng/L or pg/ml) to more readily discriminate a changing pattern. Recognizing the mounting data for the good performance of rapid protocols with hs cTn assays, the ESC published new guidelines for the management of NSTEMI patients in 2015. This update included rapid pathways (1 or 2 hours) as an alternative to the classical 0-3h protocol.2 Challenges to rapid testing were recognized, including, concerns for misdiagnosing “early presenters” (those appearing in the ED within 3 h of chest pain onset). With early presenters, a rapid protocol could lack the needed sensitivity. The authors also recognized that in patients with a high pre-test risk for MI, a changing pattern may not be seen such as those near the peak of the cTn time-concentration curve or on the downside.
Current testing guidance: The 2018 fourth Universal Definition of MI
The 2018 Fourth Universal Definition of MI (ESC/ACC/AHA/WHF Expert Consensus Document) elaborates on the use of hs-cTn assays.1 In the 6-year interim, striking progress had been made for increased commercial availability of high-sensitivity cTn assays as well as validation of these assays in both “standard” (0-3h) and “accelerated” or rapid (0-2h or 0-1h) diagnostic protocols. Differentiating acute ischemia-induced damage from cardiac injury resulting from nonischemic conditions was emphasized, as both can cause elevated cTn levels. The term myocardial injury comprises MI as well as other nonischemic cardiac conditions (such as myocarditis or heart failure) and noncardiac morbidities (such as sepsis or renal patients) associated with elevations of cTn. In the case of MI, injury is acute and characterized by a significant rise and/or fall of cTn with at least one value above the 99th percentile URL of a healthy reference population. Acute MI is diagnosed if there is evidence of myocardial necrosis (cell death due to injury) in a clinical setting consistent with myocardial ischemia. Chronic elevations are less likely to show significant change, which can aid exclusion for AMI. The 4th Universal Definition reinforces value for gender-specific cut-points. As women tend to have lower levels of cTn, the percent detection in a female reference population can be lower, meaning some assays may detect ≥50% of healthy men but not women. Additional data explored the potential for a single value rule-out using the assay limit of detection (LoD). Updates included a focus on improved diagnosis for MI types and a discussion of analytic issues for cTn, including that values from one assay cannot be applied to another due to lack of standardization.
High-sensitivity cTn: Impact on testing and patient management
Currently, hs cTn is analytically defined by the ability to detect ≥50% of a healthy reference population using values between the LoD and gender-specific 99th percentiles (with a CV <10% at the URL). The 99th percentile continues to be the recommended cut-point if using a 0-3h testing strategy, but with implementation of gender-specific values. As hs assays more accurately detect smaller levels of change, they can also be incorporated into rapid protocols (either 0-1h or 0-2h). Assay precision in these accelerated protocols is critical, as small measures of change below the 99th percentile must be reliably detected. Since hs cTn assays continue to lack standardization, each assay must be independently validated, with clinical decision limits and change values identified. The shorter the time between testing, the lower the values. Caution must be exercised depending on the hs assay utilized, as change values are not only assay-specific but can be obfuscated by low-end imprecision and lot-to-lot variation that can vary significantly among assays at values much below the 99th percentile. These and other considerations for institutions wishing to implement hs cTn testing have recently been published.3-4 Rapid protocols have been proposed to exclude patients for AMI and so reduce patient burden in the ED. High-risk patients may be more rapidly identified as well using hs assays and rapid protocols. Patients have a higher likelihood for NSTEMI if the hs-cTn concentration at presentation is at least moderately elevated, or hs-cTn concentrations show a clear rise within the first hour.
Assay-specific hs cTn: Analytic issues can impact choice of testing algorithm
The lack of standardization among cTn assays remains a challenge, necessitating assessment for the specific assay utilized in a given setting. Hs cTn assays should demonstrate ≥50% gender-specific detection in a healthy reference population. Challenges around what defines a “healthy” population exist, and screening criteria can significantly affect percent detection. Biologic variation can also contribute to divergent values, adding to the uncertainty associated with analytic variation. Any impact on low-end precision or lot-to-lot variation of hs cTn assays can confound clinical assessment when using rapid diagnostic algorithms. While all hs cTn assays meet the precision criteria at the 99th percentile, significant differences among assays exist at the lower cut-points utilized in rapid diagnostic algorithms. It is imperative that both labs and clinicians understand the precision of their assay if adopting rapid testing and not assume a low coefficient of variation (CV) extends to the lower cut-points utilized.5,6
Performance of the “classical” (0-3h) pathway with hs cTn assays
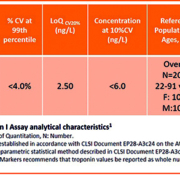
The increased sensitivity of hs cTn assays means a greater percent of chest pain patients may present with elevated values in excess of the 99th percentile. To address differentiation of elevations associated with ischemic-associated injury from alternate causes of cardiac necrosis, a 20% change value has been recommended for patients with initial elevations above the 99th and >50% of change for value below.1 Values can typically be obtained from the manufacturers package insert or published studies and percent calculated. An example listing the gender-specific 99th percentile and other assay details for a recently approved hs cTnI (Siemens Healthineers Atellica IM High-Sensitivity Troponin I) assay is shown in Table 1. Analytic performance characteristics of the Atellica IM High-Sensitivity Troponin I assay meet the criteria for an hs cTn assay.
Performance of hs cTn using rapid strategies
Rapid strategies can include employing either very low levels of hs cTn on presentation (<LoD) or the lack of significant change in persistently elevated hs-cTn values over a 1–2-hour period along with risk assessment to exclude AMI. In addition, these strategies have validated a single, high value rule-in for patients with a high index of suspicion for AMI, but again all values are assay-specific, and performance should be established in large and well-validated studies.1,5,6 A single sample rule-out strategy using a very low value has high sensitivity for myocardial injury and therefore high negative predictive value (NPV) to exclude MI, though pretest probability should be considered, along with the timing of chest-pain onset. Rapid testing strategies rely on two concepts: first, hs cTn is a quantitative and continuous variable and the probability of MI increases with increasing values; second, early absolute changes (versus relative or percent change) of cTn can be highly predictive for AMI. Importantly, morbidities such as end-stage renal disease may require an alteration of the cut-off used, though renal-specific cut-offs have yet to be widely established. Studies designed to identify cut-offs on both traditional and rapid diagnostic algorithms have often excluded patients with renal disease, as well as other co-morbidities that can be associated with cTn elevation.1 The following section reviews published data for a hs cTnI assay (Siemens Healthineers’ Atellica IM High-Sensitivity Troponin I) for use in both a traditional and rapid diagnostic algorithm. For institutions utilizing an alternate hs cTn assay, similar study guidance data is often available.
Validation studies of the 0-1h algorithm with ADVIA Centaur and Atellica IM High-Sensitivity Troponin I assays
A hs cTnI assay from Siemens Healthineers available on the ADVIA Centaur and Atellica IM analysers has been validated in three large AMI studies (one American and two European patient cohorts). The APACE study group (Advantageous Predictors of Acute Coronary Syndrome Evaluation) is an ongoing prospective international multicentre study with 12 centres in 5 European countries aiming to advance the early diagnosis of AMI. APACE investigators have validated performance for several sensitive and high-sensitive assays.7 For rapid protocols, their approach utilized a derivation cohort followed by validation for each assay studied. The results for the ADVIA Centaur High-Sensitivity Troponin I assay in a 0-1h protocol are shown in Fig. 1.
Applying the derived optimal cutoff levels and delta, 46% of patients could be classified as rule-out with a corresponding NPV of 99.7% and a sensitivity of 99.1% (using a rule-out criteria of either a single determination <3 ng/L or a 0-1h <6ng/L with a delta <3 ng/L) in patients with chest pain >3h. A single-value rule-out of 3 ng/L was applied to early presenters (chest pain <3 h from onset). Conversely, a direct rule-in based on a single ADVIA Centaur hs-cTnI concentration (≥120 ng/L) at presentation was feasible in 12% of patients, and 6% more were identified with a delta of ≥12 ng/ml at 0-1h. Overall, the 0-1h algorithm produced a diagnosis after 1 h (either rule-in or rule-out) in 64% of patients. The remaining patients (36%) underwent additional testing and observation; ultimately 11% were ruled in for NSTEMI.
To validate the 0-1h algorithm with Atellica IM High-Sensitivity Troponin I assay, two additional studies using two different cohorts have been published, one in Scotland (High-STEACS)8 and one in the U.S. (HIGH US)9. The baseline characteristics of the patients admitted at the ED are detailed in Table 2.
Importantly, Table 3 identifies key exclusion criteria differences in the testing populations. Unlike the APACE cohort, the HIGH U.S. study did not exclude renal dialysis patients, so may more closely approximate a “real world” patient testing scenario.
The High-STEACS study in Scotland validated the performance of the Atellica IM High-Sensitivity Troponin I assay in a 0-1h protocol (using the derivation values of the ADVIA Centaur High-Sensitivity Troponin I assay established with the APACE cohort); similar findings were observed with both study populations.8 The Atellica IM High-Sensitivity Troponin I assay was further validated is a US testing population (HIGH US).9 Both the ADVIA Centaur High-Sensitivity Troponin I and Atellica IM High-Sensitivity Troponin I assays utilize identical designs and differ only on the platform analyser utilized and time to result (18 minutes on ADVIA Centaur system vs. 10 minutes on Atellica IM analyser). Table 4 shows the comparable clinical performance of both the ADVIA Centaur High-Sensitivity Troponin I and Atellica IM High-Sensitivity Troponin I assays utilizing the APACE-derived values. In all three studies, a majority of patients could be excluded or diagnosed for AMI using the 0-1h strategy. Importantly, the NPV for rule-out was >99%, supporting early and safe exclusion for a significant percentage of patients across testing cohorts. The clinical accuracy for the 0-1h early rule-out of NSTEMI found with the APACE and High-STEACS cohorts was like that reported for the American cohort, despite inclusion of patients with significant renal impairments who tend to have chronic heart injury with increased cTn levels.10
Atellica IM and ADVIA Centaur High-Sensitivity Troponin I assays: Design features compatible for a fast rule-out strategy
Consistency in performance between the assays is associated with assay design, including the choice of antibodies. Three monoclonal antibodies are employed in the assay, two for the capture and one for the detection. The two monoclonal capture antibodies target unique cTnI epitopes and are conjugated to streptavidin and are preformed on magnetic latex particles to reduce interference with biotin. Specimens that contain biotin demonstrate ≤10% change in results up to 3500ng/mL. Detection of captured cTnI is accomplished using a conjugated Lite Reagent consisting of a proprietary acridinium ester and a recombinant anti-human cTnI sheep Fab covalently attached to bovine serum albumin (BSA) for chemiluminescent detection. This unique Fab has been molecularly modified to remove the primary Fc region associated with reports of human anti-animal antibodies (HAAA which can include HAMA) and other heterophile sources of interference. A direct relationship exists between the amount of troponin I present in the patient sample and the amount of relative light units (RLUs) detected by the system, producing a quantitative result. Manufacturing processes and reagent stocks have been carefully designed to provide reliable lot-to-lot consistency. Fig.2 shows the reproducibility of ADVIA Centaur High-Sensitivity Troponin I across 6 lots of reagents using a value of cTnI well below the 99th percentile (where variation would be more likely to impact clinical assessment).
Conclusion
As an alternative to the classical 0-3h protocol which now includes gender-specific 99th percentiles, a faster rule-out strategy based on a 0-1h algorithm has been validated for the Siemens Healthineers high-sensitivity cTnI assays on the ADVIA Centaur systems and Atellica IM analyser. The analytic performance of these assays supports confidence in results across the measuring range, especially at the low clinical decision cut-points. The ability to rapidly exclude a large percent of chest pain patients for AMI with a high degree of certainty can help with triage in the ED. The similar high NPV’s observed across hs cTn studies (>99%) support good clinical performance for a safe rule-out using a 0-1h strategy. The accuracy for an early AMI rule-out established by these studies supports harmonization of these algorithms worldwide for well validated assays.
References:
1) Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Circulation. 2018 Nov 13;138(20): e618-e651
2) Marco Roffi et al, 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation European Heart Journal (2016) 37, 267–315
3) Apple, FS et al. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clinical Chemistry 63:173–81 (2017)
4) Januzzi, Jr., J.L. et al. Recommendations for Institutions Transitioning to High-Sensitivity Troponin Testing. JACC Scientific Expert Panel, J Am Coll Cardiol. 2019;73(9):1059–77
5) Paul O. Collinson, Amy K. Saenger and Fred S. Apple, on behalf of the IFCC C-CBa High sensitivity, contemporary and point-of-care cardiac troponin assays: educational aids developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers Clin Chem Lab Med 2019; 57(5): 623–632
6) How Does the Analytical Quality of the High-Sensitivity Cardiac Troponin T Assay Affect the ESC Rule Out Algorithm for NSTEMI? Clinical Chemistry 65:3 (2019)
7) Boeddinghaus, J. et al. Clinical Validation of a Novel High-Sensitivity Cardiac Troponin I Assay for Early Diagnosis of Acute Myocardial Infarction. Clinical Chemistry 64:9 (2018): 1-14.
8) Andrew R Chapman et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart. 2018; 0:1–7. doi:10.1136/heartjnl-2018-314093
9) Christenson, RH et al. Trial design for assessing analytical and clinical performance of high sensitivity cardiac troponin I assays in the United States: The HIGH US study. Contemporary Clinical Trials Communications 14 (2019) 100337.
10) R.M Novak, Performance of a novel high sensitivity cardiac Troponin I assay for one Hour algorithm for evaluation of NSTEMI in the US population Journal of the American College of Cardiology Volume 73, Issue 9 Supplement 1, March 2019
The authors
Katherine Soreng, PhD is the Director of Clinical and Scientific Support for Laboratory Diagnostics at Siemens Healthineerskatherine.soreng@siemens-healthineers.com Laurent Samson, PhD, is the Associate Director for Global Commercial Marketing, Immunoassays at Siemens Healthineers laurent.samson@siemens-healthineers.com
Product availability may vary from country to country and is subject to varying regulatory requirements.