Clinical response biomarkers in ovarian cancer: current challenges and future directions
Effective screening strategies have not yet been developed for the early detection of ovarian cancer. The serum biomarker CA125, routinely used to aid diagnosis and monitor treatment response, is not informative in all patients. Recent analytical developments have prioritized promising candidate novel biomarkers or multi-biomarker panels for future clinical evaluation.
by E. L. Joseph, Dr M. J. Ferguson and Dr G. Smith
Introduction to ovarian cancer
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy and the fifth leading cause of cancer related death among women with around 140 000 annual deaths worldwide. EOC can develop as one of four histotypes with the serous histotype being the most common and most aggressive. The remaining three non-serous histotypes, endometrioid, clear cell and mucinous cancers present less frequently. High grade serous ovarian cancer, heterogeneous in nature and rapidly progressive, has a poor prognosis, where a major contributing factor is the lack of ability to diagnose the disease at a sufficiently early stage to facilitate curative surgery. The 5-year survival rate is less than 30% for patients presenting with advanced disease spread beyond the ovaries (FIGO Stage 3/4), but if detected earlier combination therapy of cytoreductive surgery and adjuvant or neo-adjuvant chemotherapy with platinum and taxane-based drugs has the potential to cure 90% of patients [1]. Consequently the identification of biomarkers capable of detecting ovarian cancer at the earliest stages and monitoring disease progression are inherently important in tackling this lethal disease. Due to its prevalence, the ideal biomarker for detecting early stage ovarian cancer requires an extremely high specificity (>99%) and a minimum sensitivity of 75% [2]. Despite extensive research, no optimal ovarian cancer biomarker has yet been identified and such high specificity is unlikely to be met by a single agent. Many promising candidate biomarkers are however currently undergoing evaluation in clinical trials.
CA125
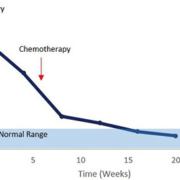
The only ovarian cancer biomarker routinely used in the clinic is cancer antigen 125 (CA125; mucin 16) currently considered the ‘gold standard’ cancer biomarker despite its limitations. In the majority of patients with EOC, expression of the CA125 glycoprotein is raised above the normal reference range (>35 U/ml blood), but it only has a sensitivity of 50% to 60% with a specificity of 90% in early stage postmenopausal patients [3]. Several factors, however, limit the utility of CA125 in routine population screening: it is not expressed in 20% of ovarian cancers, is only significantly elevated in 47% of early stage ovarian cancers (although increasing to 80–90% in advanced stage cancers) and can be raised in many benign conditions including endometriosis and peritonitis. Variability in CA125 expression throughout the menstrual cycle and in pregnancy is also a common confounding issue. A major clinical utility of CA125, however, is related to its ability to commonly reflect clinical response following chemotherapy treatment and as such is often successfully used to monitor a patient’s progress through chemotherapy. A reduction in CA125 expression during treatment is considered a positive prognostic outcome for the patient and serial serum measurements are currently used to predict therapeutic outcomes and estimate stability of the disease (Fig. 1).
Biomarkers under evaluation
There have now been multiple attempts to identify novel ovarian cancer biomarkers with varying success. The most promising serum biomarkers include HE4 and mesothelin.
HE4
Human epididymis protein 4 (HE4) has been shown to be consistently elevated above the normal level (151 pM) in ovarian cancers, with sensitivity of 95% and specificity of 73%. HE4 is differentially expressed in specific subtypes of ovarian cancer, potentially allowing clinicians to distinguish histotypes to aid treatment; HE4 was found to be overexpressed in 100% of endometrioid cancers, 93% of serous cancers but only 50% of clear cell cancers [4]. Unlike CA125, it is less likely to produce false positives in benign masses and it has also been proposed to be the best candidate biomarker for early detection of Stage I disease despite sensitivity and specificity of 46% and 95% respectively [2]. HE4 has recently obtained FDA approval in the USA for monitoring recurrence or progression of EOC and, in comparative tests, has been found to be superior to CA125 in classifying benign and borderline ovarian cancers.
Mesothelin
Mesothelin is a glycoprotein expressed by mesothelial cells, the expression of which has been found to be raised in mesothelioma, pancreatic and ovarian cancers.
It can be easily measured in both urine and serum, highlighting its potential as a non-invasive biomarker. Serum mesothelin levels were found to be increased in approximately 60% of ovarian cancers with 98% specificity.
One study found elevation of mesothelin in 42% of urine assays as opposed to 12% serum assays of early stage EOCs at 95% specificity which reinforces the potential of this glycoprotein as an early detection biomarker and the use of urine in preference to serum [2]. Higher levels of mesothelin were also found to be associated with poorer overall survival in patients following optimal debulking surgery or who have advanced stage ovarian cancer. A recent study, however, revealed that lifestyle choices such as smoking and BMI can affect mesothelin levels, which also often increase with age.
Identification of new candidate biomarkers
Due to an urgent need for better biomarkers for early detection of ovarian cancer and reliable biomarkers to monitor clinical response, ongoing efforts are focused on the application of state of the art technologies e.g. mass spectrometry and quantitative proteomic analysis to identify novel biomarkers [5]. These approaches however often generate multiple candidate biomarkers for further investigation, prioritization and clinical evaluation of which is an ongoing challenge. These methods allow comparison of multiplex biomarker panels and identification of novel differentially expressed proteins not previously linked to ovarian cancer.
Another powerful technology is microarray-based mRNA analysis which allows genome wide expression studies which have already enhanced the understanding of the genes and pathways which influence ovarian cancer progression, chemotherapy response and survival. For example, the candidate biomarkers osteopontin and kallikrein (Table 1) were discovered by this method.
Our own studies have revealed significant differences in the expression of fibroblast growth factor 1 (FGF1) and additional FGF pathway genes in ovarian cancers of different histologies (Fig. 2A) and in paired sensitive and resistant ovarian cancer cell lines (Fig. 2B). We have additionally shown that FGF1 expression is significantly inversely correlated with both progression-free (Fig. 2C) and overall survival in ovarian cancer patients [6]. We are therefore currently recruiting patients to longitudinal clinical studies to investigate whether FGF1 or additional related growth factors can predict disease progression and/or the development of treatment-limiting drug resistance.
MicroRNAs (miRNAs) are small non-coding RNAs (19–25 nucleotides) that regulate gene expression by binding to mRNA target sequences and disrupting translation [7]. MiRNAs have great potential as diagnostic and clinical response biomarkers in ovarian and additional cancers as miRNA expression can now routinely be quantitatively assessed in small biopsies and in formalin-fixed material. For example, approximately 30 miRNAs (including miR-21, miR-141, miR-203, miR-205 and miR-214) are differentially expressed in ovarian cancer [8], while miRNAs including miR-200a, miR-200b and miR-429 have also been associated with cancer recurrence and have been shown to predict survival. For example, high expression of miR-200, miR-141, miR-18a and low expression of let-7b, and miR-199a were found to predict poor survival in a cohort of 20 ovarian cancer patients [9]. Meanwhile, recent data from our own laboratory has identified multiple miRNAs including miR-125b and miR-130 associated with the development of platinum resistance. MiRNAs are particularly promising candidate biomarkers due to their stability, and abundant expression in solid cancers, whole blood and routinely collected plasma and serum samples.
Future directions
Due to the challenges of finding a single biomarker that can encompass the complexity and heterogeneity of ovarian cancer it is logical that optimization of a multi-biomarker panel may be the most practical approach, for example combining HE4 and mesothelin with CA125 to augment both sensitivity and specificity. This type of approach has recently been proposed in algorithms such as the Risk of Ovarian Malignancy Algorithm or ROMA which combines CA125 and HE4 levels with a sensitivity of 94% and specificity of 75%. [4]. Combinations of CA125 and mesothelin have also been found to detect more cancers than each biomarker alone. Several current studies have, however, suggested that combination biomarker analysis significantly increases the predictive power of CA125, but also unfortunately appears to decrease specificity. Ongoing studies therefore aim to develop improved biomarker panels suitable both for early detection and treatment guidance of ovarian cancer (Table 2). All of these results still require validation but they are indicative of the possible power of using a multi-biomarker panel in diagnostic tests and for monitoring the clinical responses of ovarian cancer.
Concluding remarks
An ideal biomarker for ovarian cancer will have a high enough sensitivity to correctly diagnose women with the disease and be specific enough to avoid false positive results. With ongoing efforts to identify biomarkers which match this ideal, hundreds of candidates with clinical relevance have been found but still require much validation before having a routine place in the clinic. It is expected that the future of ovarian cancer detection will be based on panels of combination serum-based biomarkers alongside biological imaging techniques to improve diagnosis, treatment and disease management.
References
1. Shapira I, Oswald M, Lovecchio J, Khalili H, Menzin A, Whyte J, Dos Santos L, Liang S, Bhuiya T, Keogh M, Mason C, Sultan K, Budman D, Gregersen PK, Lee AT. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer 2014; 110: 976–983.
2. Nguyen L, Cardenas-Goicoechea SJ, Gordon P, Curtin C, Momeni M, Chuang L, Fishman D. Biomarkers for early detection of ovarian cancer. Women’s Health 2013; 9: 171–185; quiz 186–187.
3. Sarojini S, Tamir A, Lim H, LI S, Zhang S, Goy A, Pecora A, Suh KS. Early detection biomarkers for ovarian cancer. J Oncol. 2012; 15.
4. Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings 2013; 3: 35–42.
5. Zhang B, Barekati Z, Kohler C, Radpour R, Asadollahi R, Holzgreve W, Zhong XY. Proteomics and biomarkers for ovarian cancer diagnosis. Ann Clin Lab Sci. 2010; 40: 218–225.
6. Smith G, NG MT, Shepherd L, Herrington CS, Gourley C, Ferguson MJ, Wolf CR. Individuality in Fgf1 expression significantly influences platinum resistance and progression-free survival in ovarian cancer. Br J Cancer 2012; 107: 1327–1336.
7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297.
8. Zhang B, Cai FF, Zhong XY. An overview of biomarkers for the ovarian cancer diagnosis. Eur J Obstet Gynecol Reprod Biol. 2011; 158: 119–123.
9. Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, Kim JW, Kim S. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008; 14: 2690–2695.
10. Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, Marks J, Godwin A, Gorelik E, Jacobs I, Menon U, LU K, Badgwell D, Bast RC, JR, Lokshin AE. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010; 28: 2159–2166.
11. SU F, Lang J, Kumar A, NG C, Hsieh B, Suchard MA, Reddy ST, Farias-Eisner R. Validation of candidate serum ovarian cancer biomarkers for early detection. Biomark Insights 2007; 2: 369–375.
12. Zhang Z, YU Y, XU F, Berchuck A, Van Haaften-Day C, Havrilesky LJ, de Bruijn HW, van der Zee AG, Woolas RP, Jacobs IJ, Skates S, Chan DW, Bast RC, Jr. Combining multiple serum tumor markers improves detection of stage I epithelial ovarian cancer. Gynecol Oncol. 2007; 107: 526–531.
13. Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT, Bigbee WL, Herberman RB, Lokshin AE. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005; 14: 981–987.
14. Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhatnaya L, Modugno F, Nolen BM, Gorelik E. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol. 2006; 102: 244–251.
The authors
Emma L. Joseph1 BSc; Michelle J. Ferguson2 MBChB, MD; and Gillian Smith1* PhD
1Division of Cancer Research, Medical Research Institute, University of Dundee, Dundee UK
2Tayside Cancer Centre, Ninewells Hospital & Medical School, Dundee UK
*Corresponding author
E-mail: g.smith@dundee.ac.uk