Detection and typing of HPV for cervical cancer prevention according to Meijer criteria
HPV testing is a linchpin of cervical cancer prevention, providing an effective alternative to the long-standing Pap test. The HPV analysis should encompass all relevant anogenital HPV types and differentiate between high-risk types, which can induce cancer, and low-risk types, which cause benign genital warts. It is also critical to identify multiple and persistent infections, since these are associated with a high tumour risk. The EUROArray HPV provides fast and reliable detection and typing of all 30 relevant anogenital high-risk and low-risk HPV types in one reaction and meets the international criteria for HPV screening defined by Meijer et al. The test is simple to perform and includes fully automated data evaluation and documentation.
by Dr Jacqueline Gosink
Cervical cancer
Cervical cancer is worldwide the third most frequent cancer in women. For example, in Germany there are approximately 4600 new cases each year, even though many women attend cancer screening. Tumours of the cervix are caused by human papillomaviruses (HPV), which are spread by sexual contact. The immune system usually eliminates the HPV within a few months. However, if an HPV infection persists over a longer period of time, this can cause changes in cervical cells, depending on the HPV type, which may subsequently lead to cancer. The cellular changes are histologically classified as grade 1, 2 or 3 cervical intraepithelial neoplasia (CIN). Mild cases (CIN 1) often clear without any treatment. Moderate and severe cases (CIN 2 and CIN 3) are usually treated to prevent development of cervical cancer.
High- and low-risk HPV types
There are over 200 types of HPV, of which 30 can cause infections in the genital area. These are divided into low-risk and high-risk types. Low-risk types cause warts in the genital area or slight tissue changes. High-risk types are significantly more aggressive. A persistent infection with a high-risk HPV type can cause tissue changes that significantly increase the risk of a tumour. The most common high-risk types are 16 and 18, which are responsible for about 70% of cervical cancers and precancers. Other high-risk types are 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82. Low-risk types are 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81 and 89.
HPV vaccination protects against infection with the high-risk types 16 and 18 and the low-risk types 6 and 11. Depending on the vaccine preparation used, protection against five additional high-risk types (31, 33, 45, 52, 58) is possible. Vaccinated persons can still become infected with other types. It is therefore important to participate in cancer screening even after vaccination.
HPV infection and cancer risk
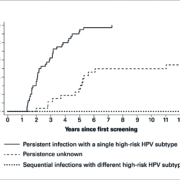
Persistent infections with a single high-risk type are associated with a clearly increased tumour risk. In a study panel (n=40), all patients with a persistent infection with a single high-risk HPV type developed CIN 2 or worse within seven years (Figure 1) (1). Furthermore, simultaneous infections with different high-risk types lead with high probability to malignant cytological changes to the cervical mucosa and therefore present a greater cancer risk for patients. Sequential infections with different high-risk HPV types do not increase the risk of cervical cancer. It is therefore important to differentiate persistent from transient infections and multiple from single infections. This is only possible with tests that are able to subtype the different HPV.
Cervical cancer screening
Cervical cancer screening is traditionally based on the Papanicolaou or Pap test, which detects morphological cell changes in cervical smear samples. However, several countries have already established or are currently switching to high-risk-HPV testing as the first-line screening method, as evidence mounts that is more effective and efficient for the prevention of invasive cervical cancer and mortality than the Pap test. Several randomized trials have shown that the cumulative incidence of cervical cancer five years after a negative HPV test is lower than the incidence three years after a normal cytology result.
Molecular biological testing allows early identification of an HPV infection, even before dysplasia is visible in the mucosa. Subtyping tests reveal at the same time whether the infection is due to low-risk or high-risk HPV and exactly which HPV types are present. Patients who have simultaneous infections with different high-risk types or a persistent infection with the same high-risk HPV type can be monitored more frequently to ensure timely treatment to minimize the risk of cervical cancer. A negative result excludes an HPV infection and thus the risk of developing cervical cancer with high probability.
Detection of the viral oncogenes E6/E7
A prerequisite for the development of carcinoma is the integration of the HPV genome into the DNA of the epidermal cells. The proportion of infected cells containing integrated viral DNA increases as the infection progresses. During the integration into human DNA, particular regions of the HPV genome (generally the E1, E2, L1 and L2 genes) are split. Test systems that detect these genes are therefore unreliable. For example, when test systems based on the L1 gene are used to detect HPV types 16 and 18, between 8% and 28% of high-grade dysplasia cases can be overlooked (2). In contrast, testing for essential viral markers such as the oncogenes E6 and E7 allows all HPV infections to be reliably detected, since these genes are essential for malignant transformation of the host cells and they remain intact even after integration. Detection of variable sequences within these genes allows the different HPV types to be differentiated.
Meijer HPV test criteria
In 2009, an international team of experts proposed criteria for the requirements and validation of HPV tests for primary cervical cancer screening, known as the Meijer criteria (3, 4). To support the clinical performance evaluation of HPV subtyping tests, the VALGENT (VALidation of HPV GENotyping Tests) protocol was subsequently established. The key issue for HPV testing in cervical screening is to detect high-risk HPV infections that are associated with or develop into ≥CIN 2 and to differentiate them from transient high-risk HPV infections. HPV tests should provide high clinical sensitivity for detection of cervical precancer and cancer, and at the same time high clinical specificity to limit unnecessary procedures and follow-up of HPV-positive women.
The validation guidelines for HPV tests encompass clinical sensitivity (criterion 1), clinical specificity (criterion 2), and intra-laboratory and inter-laboratory reproducibility (criterion 3). Candidate assays are validated by comparative analysis with fully clinically and epidemiologically validated reference HPV tests, such as the Hybrid Capture 2 (hc2) assay (QIAGEN) or the GP5+6+ PCR-EIA using samples from women aged 30 or older. One of the criteria stipulates that the sensitivity for ≥CIN 2 of the candidate assay should amount to ≥90% of the sensitivity of the hc2 assay; the specificity for ≥CIN 2 should reach ≥98% of the specificity of the hc2 assay.
EUROArray HPV
One test that fulfils the criteria of the Meijer protocol is the EUROArray HPV from EUROIMMUN. This multiplex PCR-based assay provides detection and typing of all 30 genitally relevant HPV types in one reaction. The individual typing enables differentiation of high- and low-risk infections, as well as identification of multiple infections. The precise HPV genotyping also allows differentiation between new and persistent infections when determinations are performed over a time course, e.g. two analyses at a time interval of 12 to 18 months. The test is based on detection of E6/E7 DNA, ensuring highest sensitivity even in infections where the viral genome has already integrated into the DNA of the host epithelial cells.
The EUROArray procedure is easy to perform and does not require expertise in molecular biology. DNA isolated from patient cervical smear samples is analysed using multiplex PCR and a microarray biochip slide containing DNA probes corresponding to each HPV type (Figure 2). Results are evaluated and interpreted fully automatically using the user-friendly EUROArrayScan software. A detailed result report is produced for each patient and all data is documented and archived. Integrated controls such as DNA positive control and cross-contamination control ensure high result security. Meticulously designed primers and ready-to-use PCR components further contribute to the reliability of the analysis. The entire procedure is IVD validated and CE registered.
Fulfilment of Meijer criteria
The EUROArray HPV was evaluated alongside other HPV tests (5, 6) using cervical specimens from 404 women undergoing follow-up of high-grade cytological abnormality. The HPV tests were used to detect high-risk HPV genotypes and predict histologically confirmed ≥CIN 2 in these patients. There was excellent agreement between the EUROArray HPV and all other HPV tests. The authors concluded that the EUROArray HPV fulfils the first Meijer criterion of ≥90% of the clinical sensitivity of hc2 for detection of ≥CIN 2. Moreover, the genotyping for 30 individual types would also allow the EUROArray HPV to be used in epidemiological and surveillance applications.
In a further study (7) the analytical and clinical performance of the EUROArray HPV was evaluated using a total of 1300 consecutive and 300 cytologically abnormal cervical samples (Tables 1A and 1B). The relative sensitivity of the EUROArray HPV with respect to the hc2 assay was 93% for ≥CIN 2. This value was further increased to 98% using an optimized cut-off for HPV16, which has now been incorporated into the test evaluation. The relative specificity of the EUROArray HPV for ≤CIN 1 with respect to the hc2 was 100%. Finally, the EUROArray reported excellent intra- and inter-assay reproducibility. Thus, the EUROArray HPV fulfilled all of the Meijer criteria for use in cervical cancer screening.
Conclusions
The EUROArray HPV is ideally suited for HPV genotyping in primary cervical cancer screening programmes. It has been shown to be non-inferior to the hc2 comparator test for both sensitivity and specificity, as stipulated in the Meijer criteria and validated using the VALGENT framework. The EUROArray HPV is currently the only commercially available test that enables genotyping of all 30 anogenital HPV types on the basis of the E6/E7 oncogenes. In the future it is speculated that the use of HPV genotyping in cervical cancer might be extended to testing for cure and further stratifying the disease risk. HPV are also associated with some other types of cancer, such as anal cancer, head and neck cancers, vulvar and vaginal cancers, and penile cancer. HPV testing is also important in the diagnosis of these cancers.
References
1. Elfgren et al. Am J. Obstet Gynecol (2016), 7:11-22
2. Tjalma et al. Eur J Obstet Gynecol Reprod Biol (2013), 170(1): 45-46
3. Meijer et al. Int J Cancer (2009), 124: 516-520
4. Arbyn et al. Clin Microbiol Infect (2015), 21:817-826
5. Cornall et al. Eur J Clin Microbiol Infect Dis (2016), 35(6): 1033-1036
6. Cornall et al. Papillomavirus Research (2017), 4: 79-84
7. Viti et al. J Clin Virol (2018), 108: 38-42
The author
Jacqueline Gosink, PhD
EUROIMMUN AG
Seekamp 31
23560 Lubeck
Germany