Detection of urinary microRNAs as biomarkers of diabetic kidney disease
Current measures for diagnosis and therapy of chronic kidney disease are limited. Better biomarkers are required to improve treatment by directing therapeutic intervention, tracking responses to therapy and providing greater understanding of the underlying mechanisms driving renal disease progression. We describe here the development of microRNAs as biomarkers for diabetic kidney disease, the most common etiology leading to chronic kidney disease and end-stage renal failure.
by Dr Tanya A. Smith, Dr Kate Simpson, Prof Donald J. Fraser and Dr Timothy Bowen
Diabetes, complications and biomarkers
Diabetes is a major global health challenge, with 23.1 million cases diagnosed in the US alone [1]. As described below, our laboratory is currently developing urinary microRNAs as biomarkers for diabetic kidney disease. These transcripts may also have utility as biomarkers for other complications of type 2 diabetes mellitus including diabetic retinopathy, neuropathy, cardiovascular disease, stroke, ulceration and amputation [2].
Diabetic kidney disease
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease in the United States. Clinical presentation is characterized by proteinuria, hypertension, and progressive reduction in kidney function. DKD is a progressive condition associated with around 35% of patients with type 1 and type 2 diabetes mellitus [3]. A highly significant public health concern, DKD is currently managed by targeting cardiovascular risk reduction, blood pressure management, glycemic control (hemoglobin A1c concentration), nutritional counselling, weight loss, smoking cessation, and pharmacological inhibition of the renin–angiotensin system using angiotensin-converting enzyme inhibitors or angiotensin-2 receptor blockers [4].
Despite the stabilization of the incidence of diabetes over the past 15 years, the United States Renal Data System has demonstrated increased prevalence of end-stage renal disease attributed to diabetes. However, the disease burden is such that patients often do not survive to end-stage renal disease. There is a broad spectrum of cardiovascular complications associated with DKD of which the underlying etiology remains unclear. Cardiovascular disease is the leading cause of death in this patient group, manifesting as cerebral vascular event, sudden cardiac death, myocardial infarction and diabetic cardiomyopathy. It is, therefore, essential to identify and treat patients before irreversible organ damage to reduce the medical and economic burden of disease [4].
Existing DKD biomarkers
DKD is associated with both glomerular hyperfiltration leading to progressive albuminuria, and declining glomerular filtration rate.
Albuminuria
Proteinuria is a biomarker used widely as a proxy to assess the integrity of the glomerular filtration barrier (for detailed glomerular and nephronal physiology see [5]). Quantification of urinary albuminuria excretion is a non-invasive and inexpensive method to monitor disease. Microalbuminuria is currently the primary predictive clinical DKD marker and occurs when urinary albuminuria excretion rate reaches 30–300 mg/24 h, macroalbuminuria is reached when this rate exceeds 300 mg/24 h. In the presence of diabetes mellitus, confirmation of microalbuminuria in two separate samples taken 3–6 months apart is diagnostic of DKD. Screening for albuminuria is more commonly performed using urinary albumin-to-creatinine ratio on an isolated urine sample, and is defined as >30 mg/g.
However, albuminuria is a non-specific biomarker measurable only after kidney injury has occurred and correlates poorly with clinical disease. In addition, albuminuria may be a transient DKD feature, or may occur only when widespread glomerular damage is already present [6, 7]. Recent reports have noted that up to 25% of patients with type 2 diabetes mellitus and diminished kidney function have little or no proteinuria, despite having biopsy-proven DKD [8]. There is, therefore, a need to find sensitive and specific biomarkers to predict DKD susceptibility and progression.
Estimated glomerular filtration rate
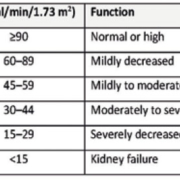
The Kidney Disease: Improving Global Outcomes (KDIGO) [4] classification is directed at adults and children over the age of 2 years old with evidence of kidney disease. Glomerular filtration rate (GFR) is considered the best measure of kidney function. Normal GFR is quantified as 100–150 ml/min and can be determined by creatinine clearance or an estimated GFR (eGFR) calculation basis on serum creatinine, age, sex and ethnicity (Table 1).
Histological features of renal biopsies, eGFR and DKD
Histological features (see [5]) correlate with functional alterations in DKD. The Renal Pathological Society system, based on glomerular changes observed in the development of DKD, groups both type 1 and type 2 diabetes mellitus patients into the four classes described below [9].
Class I: Glomerular basement membrane thickening: isolated glomerular basement membrane thickening and only mild, non-specific changes by light microscopy that do not meet the criteria of classes II–IV.
Class II: Mesangial expansion, mild (class IIa) or severe (class IIb). Glomeruli classified as mild or severe mesangial expansion but without nodular sclerosis (Kimmelstiel–Wilson lesions) or global glomerulosclerosis in >50% of glomeruli.
Class III: Nodular sclerosis (Kimmelstiel–Wilson lesions): at least one glomerulus with nodular increase in mesangial matrix (Kimmelstiel–Wilson) without changes described in class IV.
Class IV: Advanced diabetic glomerulosclerosis. Over 50% global glomerulosclerosis with other clinical or pathologic evidence showing that sclerosis is attributable to DKD.
The need for newer biomarkers
Current biomarkers do not relate well to the above pathological classification. Many potential novel biomarkers have been tested in an attempt to improve our ability to discern underlying renal pathology non-invasively, with the aim of guiding therapy. These include urinary transferrin, serum osteopontin, urinary retinol-binding protein (RBP), serum interleukin-18, serum cystatin C, serum resistin, serum TNF-α, serum interleukin-6 and urinary neutrophil gelatinase-associated lipocalin (NGAL) [reviewed in 6]. In patients with albuminuria these markers increase significantly, but their relationships with histopathological changes, eGFR, HBA1C and blood pressure is complex.
Detection and identification of microRNAs in body fluids as kidney disease biomarkers
Members of the short single-stranded endogenous RNA transcript family known as microRNAs (miRNAs) modulate the expression of most mammalian protein coding genes, thereby influencing developmental and metabolic processes, and disease phenotypes [10]. Disease-associated changes in miRNA expression profiles have been observed in cancer, cardiovascular disease, diabetes and chronic kidney disease that is treated by dialysis or transplantation [reviewed in 11–14].
To date, the majority of miRNA biomarker analyses have focused on detection of circulating transcripts [11, 13]. By contrast, the adoption into existing treatment pathways of a miRNA biomarker test on biofluid samples that can be obtained without venipuncture promises attractive reductions in time and cost [15].
We have developed RT-qPCR-based methods for precise quantification of miRNAs in urine, peritoneal dialysis effluent and renal transplantation perfusate [15–19]. The robust recovery of miRNAs from these complex analytical matrices highlights their potential utility both as non-invasive biomarkers of occurrence and/or progression of kidney disease, and as potential targets for therapeutic intervention. We have shown association of increased miR-21 with peritoneal fibrosis [17] and transplantation outcomes [18, 19]. Analysis of the renal transplantation perfusate with which the organ is supplied between donor and recipient also identified elevated miR-21 [18].
Utility of urinary miR-29b, miR-126 and miR-155 to test for DKD
Disease biomarkers are useful only when they can inform our potential to change patient treatment. The US Food and Drug Administration recommends that a reduction in eGFR of 40% over 2–3 years is a broadly acceptable effective surrogate for confirmation of CKD [20]. However, since eGFR decline is typically very gradual over the first decade or so of disease and more rapid thereafter, a biomarker that can differentiate between later stages of CKD maybe more cost-effective in detecting quantifiable responses to therapy in clinical trials [20, 21].
We have recently shown association of elevated urinary miR-29b, miR-126 and miR-155 detection predominantly in patients with type 2 diabetes mellitus and DKD [15]. We observed upregulation of these three miRNAs in two disease cohorts, obtaining an area under the curve of 0.8 in combined receiver operating characteristic curve analysis [15]. Our markers are clustered in late-stage disease (Fig. 1) and at an 80% relative quantification threshold for each miRNA, identified 48% of DKD patients with a 3.6% false positive detection rate [15]. We are currently investigating the significance of this apparent DKD patient stratification.
Utility of urinary miR-29b, miR-126 and miR-155 to investigate DKD mechanisms
We detected increased miR-29b and miR-126 in conditioned medium from cultured glomerular endothelial cells exposed to disease-related cytokines transforming growth factor-β1 and tumour necrosis factor-α, respectively [15]. It is thus conceivable that miRNAs may travel down the nephron [5] to mediate disease-related and functional effects [22]. Our data also included evidence for decreased urinary miR-192 in DKD [15], supporting our previous finding showing downregulated miR-192 expression in renal biopsies from DKD patients [23].
Conclusion
DKD is one of the most important global health challenges. Existing biomarkers provide a non-invasive approach to diagnosis and, in late-stage disease, identify the extent of kidney damage. However, there is a lack of non-invasive measures of active disease processes. New biomarkers are, therefore, required to measure risk of progressive kidney damage and to measure responses to treatment in the individual. Successful development of such biomarkers would help to individualize treatment using existing approaches, and would greatly accelerate testing of new treatments. MicroRNAs tested in urine show promise in this area.
Acknowledgments
Supported by the National Institute for Health Research Invention for Innovation (i4i) Programme grant II-LA-0712-20003 and Kidney Research UK Project grant award RP44/2014. The Wales Kidney Research Unit is funded by core support from Health and Care Research Wales.
Disclosure
TB and DF are inventors for patent WO/2017/129977 Chronic Kidney Disease Diagnostic.
References
1. National diabetes statistics report, 2017: estimates of diabetes and its burden in the United States. Centers for Disease Control and Prevention (CDC) 2017 (https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf).
2. Wang J, Chen J, Sen S. MicroRNAs as biomarkers and diagnostics. J Cell Physiol 2016; 231(1): 25–30.
3. de Boer IH, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305(24): 2532–2539.
4. Levin A, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Supplements 2013; 3(1): 1–150.
5. Pollak MR, et al. The glomerulus: the sphere of influence. Clin J Am Soc Nephrol 2017; 9(8): 1461–1469.
6. Al-Rubeaan K, et al. Assessment of the diagnostic value of different biomarkers in relation to various stages of diabetic nephropathy in type 2 diabetic patients. Sci Rep 2017; 7(1): 2684.
7. Alicic RZ, et al. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12(12): 2032–2045.
8. Dwyer JP, Lewis JB. Nonproteinuric diabetic nephropathy: when diabetics don’t read the textbook. Med Clin North Am 2013; 97(1): 53–58.
9. Tervaert TW, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21(4): 556–563.
10. Bartel DP. Metazoan microRNAs. Cell 2018; 173(1): 20–51.
11. Simpson K, et al. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diab Rep 2016; 16(3): 35.
12. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2016; 16(3): 203–222.
13. Wonnacott A, et al. MicroRNAs as biomarkers in chronic kidney disease. Curr Opin in Nephrol and Hypertens 2017; 26(6): 460–466.
14. Zhao H, et al. MicroRNAs in chronic kidney disease. Clin Chim Acta 2019; 491(4): 59–65.
15. Beltrami C, et al. Association of elevated urinary miR-126, miR-155 and miR-29b with diabetic kidney disease. Am J Pathol 2018; 188(9): 1982–1992.
16. Beltrami C, et al. Stabilization of urinary microRNAs by association with exosomes and argonaute 2 protein. Noncoding RNA 2015; 1(2): 151–165.
17. Lopez Anton M, et al. MicroRNA-21 promotes fibrogenesis in peritoneal dialysis. Am J Pathol 2017; 187(7): 1537–1550.
18. Khalid U, et al. MicroRNA-21 (miR-21) expression in hypothermic machine perfusate may be predictive of early outcomes in kidney transplantation. Clinical Transplant 2016; 30(2): 99–104.
19. Khalid U, et al. A urinary microRNA panel that is an early predictive biomarker of delayed graft function following kidney transplantation. Sci Rep 2019; 9: 3584.
20. Levey AS, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014; 64(6): 821–835.
21. Stevens LA, et al. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 2006; 1(12): 874–884.
22. Thomas MJ, et al. Biogenesis, stabilization and transport of microRNAs in kidney Health and Disease. Noncoding RNA 2018; 4(4): E30.
23. Krupa A, et al. Loss of microRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 2010; 21(3): 438–447.
The authors
Tanya A. Smith MB ChB; Kate Simpson PhD; Donald J. Fraser MB ChB, PhD; Timothy Bowen* PhD
Wales Kidney Research Unit, Cardiff University School of Medicine, Cardiff, CF14 4XN, UK
*Corresponding author
E-mail: bowent@cardiff.ac.uk