Determination of methylphenidate and ritalinic acid in serum and saliva of patients with ADHD
by Sophie Studer, Hans-Willi Clement, Christian Fleischhaker, Eberhard Schulz
Attention deficit hyperactivity disorder (ADHD)
What do fidgets and Johnny Head-in-the-Clouds (a fictional character from a German tale) have in common with Alexander the Great, Winston Churchill or Benjamin Franklin? For all of these, a diagnosis of ADHD would be made today [1]. The first indications of behavioural abnormalities in childhood date back to the mid-19th century. However, clear descriptions of the medical condition were first found in 1902 in the notes of the English pediatrician George Still. The characteristics he described were extreme motor unrest and “the abnormal inability to maintain concentration”, which led to failure to achieve at school. In 1932, two neurologists at the Berlin Charité Hospital, Kramer and Pollnow, described the symptoms of an illness they termed a “hyperkinetic disease”, which included the inability to appreciate danger, to follow rules, to control impulses and a lack of planning skills [2], as well as being easily distracted and showing motor hyperactivity. This was the first description of the leading symptoms of ADHD in German language, which is still valid – hyperactivity, inattentiveness and impulse control disorder. In order to be able to evaluate these characteristics and to investigate hyperactivity symptoms in a standardized way, Conners developed parent and teacher questionnaires at the end of the 60s that are still used today [3].
Most scientific papers are merely limited to attempts to explain the origin and course of the disease portrayed. Whereas these hyperactivity symptoms are actually seen to be the interaction of morphological changes already present at birth with external factors that affect the organism.
Methylphenidate (Ritalin®)
Today, stimulants such as Ritalin® in combination with psychotherapy and psychoeducation represent the method of choice for the treatment of hyperkinetic disorders. When a definite diagnosis has been made, pharmacotherapy is always indicated if the ADHD symptoms are marked, occur in many situations and when the effectiveness or practicability of psychoeducative and behavioural therapy measures are lacking. In addition, no contraindications for the individual psychostimulants must exist.
Methylphenidate (MPH) demonstrably improves the core symptoms of ADHD [4] and is one of the best-researched pediatric psychopharmaceuticals with long-term clinical experience. Nevertheless, the “pill for the troublemaker” is one of the most controversially discussed pharmacological products. A frequently mentioned point is the possible addiction potential of Ritalin®, for which reason the drug is also subject to the German controlled substances act. However, one must differentiate here between oral administration in therapeutic doses and “snorting” or intravenous application in excessive amounts.
The story of Ritalin® begins at the Swiss company Ciba, where the psychostimulant was successfully synthesized and the effectiveness of the substance proven in a self-experiment. When the drug was taken by Leandro Panizzon’s wife Marguerite (“Rita”), she made considerable progress. Ritalin®, probably the best-known MPH today, is named after her. Ciba introduced it to the market in 1954, 10 years after its development, for the treatment of psychoses, chronic tiredness and lethargy [5]. A short time later, meta-analyses became possible based on numerous study results. A distinct alleviation of symptoms was shown in about 75 % of all children treated with Ritalin® for ADHD. Alongside the reduction of hyperactivity and impulsiveness in the mentioned group, the ability for concentration and attentiveness increased considerably, also manifested in improved school achievements [6-8].
Structure and metabolism of methylphenidate
The fundamental structure of MPH is based on the phenylethylamine skeleton (Fig. 1a) and exhibits no hydroxyl group on the phenyl ring, facilitating diffusion into the central nervous system. It exhibits two chiral centres, consequently there are four configuration isomers (Fig. 1b). In practice, only the D- and L-threo forms find use in the treatment of ADHD. In the USA and in Switzerland the pure D- threo dextromethylphenidate isomer (Focalin®) is approved, and is regarded as the main pharmacologically active form. In comparison, the original Ritalin® consists of a mixture of the enantiomeric D- and L-threo forms. MPH is always manufactured in the protonated form as the hydrochloride salt [5].
The oral bioavailability of MPH is about 30 % (D-enantiomer > L-enantiomer), whereby foodstuffs have no relevant influence on the resorption. Generally available preparations reach their maximum plasma level within 1.5-2 hours. The effect is already shown after 15-30 minutes and reaches its highest level after 2-3 hours. In contrast, retard preparations such as Concerta® have a considerably longer duration of effect, which can be around 10-12 hours.
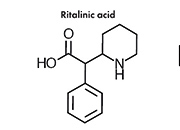
MPH is rapidly metabolized renally by carboxylesterase CES1A1 to pharmacologically inactive 2-phenyl-2-(piperidin-2-yl) acetic acid (ritalinic acid, RA). The maximum plasma level of the metabolite is 30-50 times greater than that of the original drug and the half-life is about twice as long. However, as RA possesses only a small pharmacodynamic activity, or none at all, this fact is of minor significance.
TDM of Ritalin® in children
For monitoring pharmacotherapy through concentration measurements, the collection of blood has so far been unavoidable. However, invasive methods present a compliance obstacle, particularly for children. Therefore, to ensure a high degree of drug safety, a method based on alternative body fluids for TDM is desirable. Saliva is becoming increasingly significant in this respect and is already being investigated routinely in immunology and infectious serology diagnostics, in drug and drug-abuse screening and for determining levels of the hormone cortisol [9].
The research group of Marchei et al. has already successfully developed saliva diagnostics for MPH and RA using LC-MS/MS [10]. Further investigations demonstrated almost parallel changes in the MPH and RA concentrations over the time in serum and saliva [11].
These facts, and the availability of the MassTox® TDM Series A Kit from Chromsystems, which permits the determination of the psychostimulant methylphenidate and its metabolites in serum/plasma, were the starting point for an investigation into an LC MS/MS method from Chromsystems for the determination of these analytes in saliva.
For this, serum and saliva samples from 19 ADHD patients (nine children, one adolescent and nine adults) being treated with MPH were collected and investigated. The study participants mainly took long-acting retard products, such as Medikinet retard® or Ritalin LA®. The daily intake ranged from 5 to 60 mg of MPH, corresponding to a dosage of 0.11 to 1.43 mg MPH per kilogram body weight.
As part of the routine follow-up investigations, serum was obtained by blood collection using a serum Monovette, two hours after administration of the drug where possible. In parallel, saliva samples were obtained from the patients using the Salivette system. For this, the subjects chewed on a cotton swab for 2-5 minutes during blood collection. The samples were centrifuged immediately afterwards, aliquoted and then shock frozen in liquid nitrogen to avoid degradation of the substances to be analysed.
Materials and methods
Kit for LC-MS/MS analysis: MassTox® TDM Antidepressants 2/Psychostimulants (atomoxetine, methylphenidate, mianserin, reboxetine, ritalinic acid, trazodone; (Chromsystems GmbH), methylphenidate hydrochloride C-II (Sigma-Aldrich), saliva (IBL Hamburg).
After a brief storage at -80°C, the serum and saliva samples were processed using the parameter set for Antidepressants 2/Psychostimulants for LC-MS/MS analysis and following the manufacturer’s instructions (Table 1). The calibrators and control materials for the determination of MPH in serum/plasma were also from Chromsystems. To produce a series of MPH standards in saliva, saliva (IBL Hamburg) was spiked with MPH hydrochloride (Sigma-Aldrich).
After sample preparation, the eluates obtained were separated chromatographically in an analytical column at a flow rate of 0.6 ml/min (MasterColumn® A, Chromsystems) and then quantified in a mass spectrometer (Thermo TSQ Quantum Ultra) according to their mass-to-charge ratio (Fig. 2).
The Chromsystems test is approved for the determination of psychostimulants in serum/plasma. Figure 3A shows the chromatogram of a patient who has taken Medikinet adult® at a dosage of 30 mg per day. The determination in serum gave a value of 5.5 ng/ml for MPH and 195 ng/ml for RA. As was to be expected from data in the literature, the values for the determination of MHP in saliva were considerably higher – in this case by a factor of 4 – whereas considerably lower values were determined for RA (Fig. 3B) [12].
A comprehensive verification of the determination of MPH and its acid metabolite is still to be performed. Nevertheless, initial experiments to determine the variance within a preliminary inter-assay study have already been carried out. The results are summarized in Table 2.
The values measured for saliva were only slightly poorer than the values for MPH in serum, also determined with the
MassTox® TDM Parameter Set Antidepressants 2/ Psychostimulants.
Conclusions
In order to carry out an effective pharmacotherapy with few side effects, it is necessary to establish less-invasive TDM methods. This applies to sensitive patient groups, such as children and adolescents who display distinctly different pharmacokinetic characteristics, indicating the need for a much tighter monitoring of compliance [13]. A similar situation in respect of altered metabolic characteristics can be found in patients with liver or kidney failure, who would also benefit from a less-invasive sample collection method. In summary, the data described here have shown the methodological and analytical suitability of the MassTox® TDM Series A – PARAMETER Set Antidepressants 2/
Psychostimulants in serum/plasma (Chromsystems) – for the determination of MPH and its metabolite RA by LC-MS/MS in both serum/plasma and in saliva. Thus facilitating a much more simplified way of drug monitoring in this special case of pharmacotherapy.
References
1. Krause J, Krause KH. ADHS im Erwachsenenalter. Die Aufmerksamkeitsdefizit-/ Hyperaktivitätsstörung bei Erwachsenen. 3. Aufl, Schattauer Verlag Stuttgart (2009).
2. Kramer F, Pollnow H. (1932) Über eine hyperkinetische Erkrankung im Kindesalter. Monatsschrift für Psychiatrie und Neurologie 82(1-2): 1-40.
3. Steinhausen HC. Der Verlauf hyperkinetischer Störungen. In: Steinhausen HC (Hrsg). Hyperkinetische Störungen im Kindes- und Jugendalter. Kohlhammer Verlag Stuttgart (1995).
4. Riederer P, Batra A. Neuro-Psychopharmaka. Ein Therapie-Handbuch. 2. neu bearbeitete Aufl, Springerverlag Berlin, Heidelberg (2006).
5. Kappeler T. (2007) Methylphenidat: Basics für die Apotheke. pharmaJournal 10: 4-7.
6. Kavale K. (1982) The efficacy of stimulant drug treatment for hyperactivity: a meta-analysis. J Learn Disabil 15(5): 280-9.
7. Schachter HM, Pham B, King J, Langford S. (2001) How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit. CMAJ 165(11): 1475-88.
8. Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D. (1996) Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 35(4): 409-32.
9. Chiappin S, Antonelli G, Gatti R, De Palo EF. (2007) Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 383(1-2): 30-40.
10. Marchei E, Farrè M, Pellegrini M, Rossi S, García-Algar Ó, Vall O, Pichini S. (2009) Liquid chromatography–electrospray ionization mass spectrometry determination of methylphenidate and ritalinic acid in conventional and non-conventional biological matrices. J Pharm Biomed Anal 49(2): 434-9.
11. Marchei E, Farrè M, Garcia-Algar O, Pardo R, Pellegrini M. (2010a) Correlation between methylphenidate and ritalinic acid concentrations in oral fluid and plasma. Clin Chem 56(4): 585-92.
12. Marchei E, Farrè M, Pellegrini M, Rossi S, García-Algar Ó, Vall O, Pacifici R, Pichini S. (2010b) Pharmacokinetics of methylphenidate in oral fluid and sweat of a pediatric subject. Forensic Sci Int 196(1-3): 59-63.
13. van den Anker JN, Schwab M, Kearns GL. (2011) Developmental pharmacokinetics. Handbook of experimental pharmacology 205: 51-75.
The authors
Sophie Studer, Hans-Willi Clement, Christian Fleischhaker, Eberhard Schulz
University Hospital Freiburg, Department of Child and Adolescent Psychiatry, Psychotherapy and Psychosomatics, Neuropharmacological Research Laboratory, Freiburg, Germany