Do point-of-care cardiac troponin assays perform sufficiently well to achieve clinical guidelines to rule in or to rule out acute myocardial infarction?
Current emergency department strategies are aimed at reliably excluding myocardial infarction as soon as possible through clinical assessment and time-dependent measurement of high-sensitivity cardiac troponin. Point-of-care cardiac troponin methods have evolved, but can they be used to support the early rule-in or rule-out strategies for myocardial infarction?
by Dr Martha E. Lyon and Dr Andrew W. Lyon
Introduction
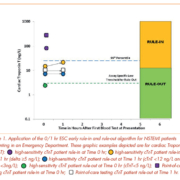
Significant attention has recently focused on early rule-in and rule-out strategies to detect non-ST-segment elevation (NSTEMI) acute myocardial infarction in the emergency department (ED) [1]. In 2015, the European Society of Cardiology (ESC) introduced guidelines for the management of acute coronary syndrome in patients without ST-segment elevation [2]. This guideline included interpretative algorithms to rule in or rule out acute myocardial infarction (AMI) based on clinical symptoms, high-sensitivity cardiac troponin (hs-cTn) concentrations at specific thresholds and changes in hs-cTn over intervals of 1 or 3 hours (Fig. 1) [2]. Importantly, the guidelines also highlighted the time-dependent uncertainty of using low concentration cut-offs in patients presenting early after the onset of pain with the following comment, “Only applicable if chest pain onset <3 h.” hs-cTn methods used in hospital clinical laboratories are expected to have an imprecision of ≤10% at the 99th percentile of a healthy population and allow for the detection of at least 50% and ideally >95% of healthy individuals [3]. The analytical qualities of the high-sensitivity methodology enable excellent diagnostic performance, that being a 99% clinical sensitivity and negative predictive value. However, it should be acknowledged that the prevalence of AMI in a specific population and the clinical sensitivity of the cardiac troponin test will influence the calculation of the negative predictive value [1]. Physicians will need to confirm that clinical trial populations are representative of their local population in order to verify the applicability of the diagnostic performance of the hs-cTn method [1].
Many studies with hs-cTn methods have investigated the derivation of upper reference limits, rates of change in hs-cTn concentration to detect AMI, assay imprecision at the 99th percentile concentration and performance characteristics of commercial assays with various interpretative thresholds [2, 4, 5]. Additional factors such as hemolysis, anticoagulant-bias, and within-subject variation will cause bias and imprecision in method results [6, 7]. An understanding of the interaction between these factors is incomplete because of the limited sample size in many clinical studies and the poor correlation between commercial assays [8]. In an initial attempt to understand this complex and complicated interaction, we used computer simulation models to predict the influence of method bias and imprecision on the rates of misclassification at low interpretative thresholds to rule out AMI and at thresholds near or exceeding the overall 99th percentile to rule in AMI. We found that at low thresholds, only method bias and not imprecision influenced the rate of misclassification whereas both method bias and imprecision would affect the early rule-in for AMI [9].
Point-of-care (POC) cardiac troponin devices
Short turnaround testing (STAT) in central hospital laboratories commonly employs the expectation of 1-hour turnaround time once the specimen has arrived in the laboratory. The ability to consistently meet the earlier time points, as outlined in the guideline algorithms, will represent a logistic challenge for many hospitals. POC methods provide an appealing alternative to central laboratory assessment, in particular for the initial rule-in when elevated cTn levels are present at Time 0 hr as well as sequential monitoring. However, prior to implementing a POC troponin method, comparison studies between the POC method and central laboratory cardiac troponin methods need to be conducted to assure concordance of the results. Several studies have reported a significant gap in the analytical sensitivity between hs-cTn and POC troponin methods [10, 11]. In 2015, Amundson and Apple described the analytical performance characteristics of POC cardiac troponin methods from nine different manufacturers [12]. These characteristics included clinical sensitivity and specificity, analytical imprecision, specimen type and preparation as well as the method principle of analysis. Although each of the devices provided different qualities, it was determined that an imprecision of ≤20% at the 99th percentile was paramount to limit both false-positive and false-negative results [12].
Accurate and precise measurement of cardiac troponin is essential for the consistent identification of NSTEMI patients with acute coronary syndrome. Currently, significant variation exists between clinical laboratory hs-cTn methods that could influence the clinical care provided to patients [8]. This also represents a challenge to the adoption of POC cTn technology.
Simulation models
Computer simulation model utility has been demonstrated with investigations of the impact of method bias and imprecision on potential clinical risk of insulin dosing errors with glucose meters [13], warfarin dosing errors with POC international normalized ratio (INR) devices [14] and with early rule-in and rule-out of AMI misclassification rates with cTn methods [9]. Clinical studies are challenging to conduct with NSTEMI patients presenting early in an emergency department because of variation in disease prevalence, poor correlation between cTn analytical methods and uncertainty in the time of pain onset. Low prevalence and variation in disease over time are common problems with other clinical laboratory biomarkers such as anti-viral antibodies as well as first-trimester pregnancy screening methods [15, 16]. One solution to this concern has been to generate finite mixture models of biomarker distributions to predict biomarker assay performance [17]. These simulated databases have been used to understand the relationship between clinical risk and assay characteristics and to extend use of the statistical information gathered in small clinical trials.
Simulation model investigation of the utility of POC cardiac troponin testing
Recognizing the lack of hs-cTn method standardization, the low incidence of NSTEMI AMI and the high cost of conducting clinical trials, few studies have assessed the utility of POC cardiac troponin methods to rule-in or rule-out AMI. To overcome these limitations, we recently used a simulation model to predict the diagnostic performance of two POC troponin methods (Radiometer AQT90 and Roche cobas h 232) relative to a hs-cTnT method (Roche cobas 6000/Elecsys) using an Emergency Department patient database proportionately expanded to n=10 000 in a finite mixture model. This study was presented at the 2017 Annual Conference for the American Association of Clinical Chemistry and Canadian Society of Clinical Chemists [18]. Finite mixture analysis of the 0-hr data obtained from the ROMI trial (n=1137 Optimal Troponin Cut-Offs for acute coronary syndrome by Roche hs-cTnT) enabled derivation of a simulation data set (n=10 000) troponin test results. Published regression equations were used to convert the hs-cTNT results into simulated AQT90 and h 232 cTnT results [19, 20]. Clinical sensitivity, specificity, positive and negative predicative values were calculated using the simulated hs-cTnT, AQT90 and h 232 data for AMI diagnosis using the limit of detection for the assays (Table 1).
The Roche hs-cTnT in this simulated data set achieved both sensitivity and negative predictive values above 99%. The predicted performance of the Radiometer AQT90 cTnT POC assay approached the estimates for hs-cTnT, suggesting POC methods are emerging that could be used both for AMI rule-in and AMI rule-out. The high limit of detection of the h 232 POC method limited its sensitivity and negative predictive values.
Conclusions
Rapid and reliable measurements of cardiac troponins are ongoing analytical challenges in laboratory medicine because this clinical tool is being used both to rule in and rule out NSTEMI. Clinical trials will be required to prospectively measure the diagnostic utility of high-sensitivity central laboratory and novel POC cTn methods, but simulation studies provide useful predictions. Our recent simulation study predicted we are now in an age where POC cTn methods are approaching analytical performance necessary to effectively rule in and rule out NSTEMI.
References
1. Morrow DA. Clinician’s guide to early rule-out strategies with high sensitivity cardiac troponin. Circulation 2017; 135: 1612–1616.
2. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315.
3. Jarolim P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin Chem Lab Med 2015; 53: 635–652.
4. Wildi K, Gimenez MR, Twerenbold R, Reichlin T, Jaeger C, Heinzelmann A, Arnold C, Nelles B, Druey S et al. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation 2015; 131: 2032–2040.
5. Love SA, Sandoval Y, Smith SW, Nicholson J, Cao J, Ler R, Schulz K, Apple FS. Incidence of undetectable, measurable, and increased cardiac troponin I concentrations above the 99th percentile using a high-sensitivity vs a contemporary assay in patients presenting to the emergency department. Clin Chem 2016; 62: 1115–1119.
6. Krintus M, Kozinski M, Boudry P, Capell NE, Koller U, Lackner K, Lefèvre G, Lennartz L, Lotz J et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med 2014; 52: 1657–1665.
7. Ryan JB, Wallace J, Sies CV, Florkowski CM, George PM. Evaluation of Abbott Architect high-sensitivity troponin I assay for haemolysis interference. Pathology 2015; 47: 716–718.
8. Ungerer JPJ, Tate J, Pretorius JC. Discordance with 3 cardiac troponin I and T assays: implications for the 99th percentile cut-off. Clin Chem 2016; 62: 1106–1114.
9. Lyon AW, Kavsak P, Lyon OAS, Worster A, Lyon ME. Simulation models of misclassification error for single thresholds of high-sensitivity cardiac troponin I due to assay bias and imprecision. Clin Chem 2017; 63: 585–592.
10. Palamalai V, Murakami MM, Apple FS. Diagnostic performance of four point of care troponin I assays to rule in and rule out acute myocardial infarction. Clin Biochem 2013; 46: 1631–1635.
11. Bruins Slot MHE, van der Heijden GJMG, Stelpstra SD, Hoes AW, Rutten FH. Point-of-care tests in suspected acute myocardial infarction: A systematic review. Int J Cardiol 2013; 168: 5255–5262.
12. Amundson BE, Apple FS. Cardiac troponin assays: a review of quantitative point-of-care devices and their efficacy in the diagnosis of myocardial infarction. Clin Chem Lab Med 2015; 53: 665–676.
13. Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin Chem 2010; 56: 1091–1097.
14. Lyon ME, Sinha R, Lyon OAS, Lyon AW. Application of a simulation model to estimate treatment error and clinical risk derived from point-of-care INR device analytic performance. J Appl Lab Med 2017; 2: 25–32.
15. Harelip P, Williams D, Dezateux C, Tookey PA, Peckham CS. Analysis of rubella antibody distribution from newborn dried blood spots using finite mixture models. Epidemiol Infect 2008; 136: 1698–1706.
16. Wright D, Abele H, Baker A, Kagan KO. Impact of bias in serum free beta-human chorionic gonadotroponin and pregnancy-associated plasma protein-A multiples of the median levels on first-trimester screening of trisomy 21. Ultrasound Obstet Gynecol 2011; 38: 309–313.
17. Deb P, Trivedi PK. Demand for medical care by the elderly: a finite mixture approach. J Appl Econ 1997; 12: 313–326.
18. Lyon ME, Kavsak PA, Worster A, Lyon AW. Simulation models to rule out acute myocardial infarction with two point-of-care testing devices and a high sensitivity cardiac troponin T method. Poster Presentation. AACC/CSCC Annual Meeting, San Diego, CA, USA, 2017.
19. Bertsch T, Chapelle JP, Dempfle CE, Giannitsis E, Schwab M, Zerback R. Multicentre analytical evaluation of a new point-of-care system for the determination of cardiac and thromboembolic markers. Clin Lab 2010; 56: 37–49.
20. Le Goff C, Evrards S, Brevers E, Kaux JF, Cavalier E. Evaluation of troponin T on AQT90 and Cobas 8000 as a rule-in/-out tool in an emergency ward. Poster Presentation, EuroMed Lab Conference 2014.
The authors
Martha E. Lyon* PhD, DABCC, FACB and Andrew W. Lyon PhD
Department of Pathology & Laboratory Medicine, Division of Clinical Biochemistry, Saskatoon Health Region, Saskatoon, Saskatchewan, Canada
*Corresponding author
E-mail: martha.lyon@saskatoonhealthregion.ca