Establishing flow cytometry as a primary diagnostic method for the investigation of suspected platelet function disorders
Although considerable progress has been made in our understanding of the role of platelets in hemostasis, the analytical methods clinically available for investigating platelet function defects remain limited. Herein, we describe an initiative at Linköping University Hospital, Sweden, to use flow cytometry for measuring platelet function in patients with a suspected bleeding disorder.
by Dr Niklas Boknäs, Dr Sofia Ramström and Prof. Tomas Lindahl
Introduction
Although many patients seek professional help for bleeding problems, very few end up receiving an informative diagnosis, even when the presenting symptoms are clearly abnormal [1]. At present, our diagnostic tools for the investigation of bleeding symptoms are tailored for identifying serious disorders with dramatic symptoms such as hemophilia and Glanzmann’s thrombastenia, but often fail to identify the underlying defect in mild bleeding disorders (MBD) [2]. Ironically, the reverse is also often true, as the clinical significance of many tests performed during conventional laboratory investigations of MBDs is ill-defined [3].
Platelet function disorders (PFDs) represent a subcategory of MBDs where the underlying hemostatic defect is caused by abnormally low platelet pro-hemostatic activity. As PFDs produce virtually identical clinical symptoms to many other conditions causing bleeding problems, diagnosing PFDs necessitates access to reliable laboratory testing of platelet function. Ideally, such tests could provide important guidance in a number of clinical situations, such as when deciding on whether to give pharmaceutical prophylaxis in the event of frequent bleeding or surgery and when assessing the risks associated with the use of thromboprophylaxis after thrombosis and surgery in the individual patient.
Unfortunately, clinical tests evaluating platelet function have evolved poorly during recent decades, despite the introduction of new promising techniques. Light transmission aggregometry (LTA), the method currently considered gold standard for evaluating platelet function, has been used for more than five decades and comprises continuous measurement of the optical density of stirred platelet-rich plasma after stimulation with agonists. LTA gives information about how platelets aggregate upon stimulation, but does not enable measurement of other aspects of platelet pro-hemostatic activity such as platelet adhesion, granule secretion and alterations of platelet membrane structure to accelerate coagulation. From our experience, the clinical value of LTA in terms of explaining patient symptoms is limited, and this is supported by studies failing to show an association between results from LTA and the severity of bleeding problems among patients with MBD [1, 4]. In addition to this limitation, LTA remains poorly standardized and labour-intensive, making performance of LTA only feasible in specialized hemostasis laboratories.
Flow cytometry for the diagnosis of PFD in patients with MBD
In an effort to overcome these problems with the methods currently used for diagnosing PFD, we and others have switched to employing whole-blood flow cytometry for the diagnosis of PFD among patients with MBD. Whole-blood flow cytometry for platelet function testing (FC-PFT) was developed in the 1980s [5, 6]. A description of the analytical principle behind flow cytometry is outside the scope of this article, but in this context, the technique can extremely briefly be described as a powerful method to quantify the presence of different epitopes on the surface of platelets after platelet activation by the use of fluorescent probes that bind to the cell surface. Compared to LTA, FC-PFT confers the following practical advantages [7]:
- Samples can be analysed in anticoagulated whole blood, eliminating the need for pre-analytical manipulation of blood components.
- Sample volumes can be reduced drastically, which is especially advantageous in children.
- Results are not influenced by platelet count, enabling assessment of platelet function in patients with thrombocytopenia [8].
- The work load is reduced considerably as many samples can be analysed in rapid sequence.
- Flow cytometry is a very common technique, and appropriate instruments are widely available in most clinical and research laboratories.
In addition to these practical benefits with FC-PFT, the method confers several other advantages. For example, it produces numerical results that are easy to interpret, and can give information about several different aspects of platelet activation by the employment of different fluorescent probes detecting distinct events during platelet activation [9]. The ability to measure different aspects of platelet function also allows the direct diagnosis of rare disorders, such as Bernard-Soulier syndrome, Glanzmann’s thrombastenia and Scott syndrome, without the need for sequential testing [10].
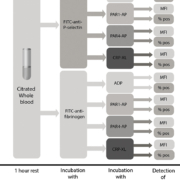
Unfortunately, until recently no studies had addressed the clinical utility of FC-PFT for diagnosing clinically relevant PFDs. To address this issue, we recently published a clinical study comparing the results from FC-PFT with symptom severity in a cohort of bleeders [11]. The study was performed on 105 patients referred to Linköping University for evaluation of platelet function. Only patients wherein a complete diagnostic work-up including a full blood cell count, APTT (activated partial thromboplastin time), PT (prothrombin time), FVIII (factor 8) and von Willebrand factor (antigen and ristocetin cofactor activity) had excluded the presence of von Willebrand disease or a coagulation disorder were included in the study. Bleeding symptoms were assessed by a single experienced clinician blinded to the laboratory results of the study. In our panel for FC-PFT, we included analysis of fibrinogen binding (indicating activation of the fibrinogen receptor glycoprotein (GP)IIb/IIIa responsible for platelet aggregations) as well as P-selectin exposure (indicating release of platelet alpha granules) after platelet stimulation with a panel of four different agonists that specifically activate the most important platelet receptors: P2Y12 and P2Y1 (ADP); the thrombin receptors PAR1 and PAR4 [PAR1-activating peptide (AP), PAR4-AP]; and the collagen receptor GPVI (CRP-XL). To assess the contribution of dense granules to platelet activation, we designed an indirect test wherein the effects of pre-incubation with apyrase (which degrades ADP) was used as a measure of functional dense granule release. A flow chart illustrating the flow cytometry protocol is provided in Figure 1.
Our results clearly demonstrate that abnormal test results using FC-PFT are associated with a more severe bleeding phenotype in patients with MBDs. In fact, a high symptom burden was 5–8 times more common among patients with more than two abnormal test results in our study as compared to patients with two or fewer abnormal test results (Fig. 2), depending on which method that was used for calculating the reference range for the different tests. When results pertaining to the fifth percentile of the patient material was classified as abnormal and more than two abnormal test results were used as a predictor for bleeding symptom severity, a high symptom burden was predicted with as specificity of 95 % and a positive predictive value of 80 %. It should be noted however, that the clinical material was insufficient to allow for a prospective validation of these estimates in a separate patient cohort.
Discussion
In our opinion, FC-PFT for clinical use should as a minimum comprise: (a) testing of platelet integrin activation, either directly by the use of the anti-PAC-1 antibody (recognizing GPIIb/IIIa) or indirectly by measuring fibrinogen binding or microaggregate formation; (b) a marker of alpha granule secretion, preferably by using an antibody directed towards P-selectin; and (c) a test of dense granule secretion to accurately assess the clinically most important hemostatic functions of platelets. Ideally, a clinical protocol for FC-PFT should also include a marker of platelet procoagulant platelet activity and a fluorescent marker binding to GPIbα, in order to provide a more complete assessment of the platelet hemostatic repertoire and diagnose the rare hereditary disorders Scott syndrome and Bernard-Soulier syndrome. In our own protocol, we have recently incorporated these two additional functionalities. We have also improved our protocol by incorporating the use of fixatives and pre-preparation of frozen reagents in order to improve reproducibility and increase the time- and cost-efficiency of the protocol. Recently, very promising methodological improvements have been made by other researchers, such as the use of fluorescent beads as an internal control for standardizing results and facilitating comparisons between different instruments [12] and the use of a modular diagnostic algorithm to ensure efficient and exact diagnosis [13]. Thus, continuous efforts are being made to firmly establish FC-PFT as an attractive alternative for platelet function testing in the setting of MBDs.
References
1. Quiroga T, Goycoolea M, Panes O, Aranda E, Martínez C, Belmont S, Muñoz B, Zúñiga P, Pereira J, Mezzano D. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica 2007; 92(3): 357–365.
2. Quiroga T, Mezzano D. Is my patient a bleeder? A diagnostic framework for mild bleeding disorders. ASH Educ Progr B 2012; 2012(1): 466–474.
3. Harrison P. Platelet function analysis. Blood Rev 2005; 19(2): 111–123.
4. Lowe GC, Lordkipanidzé M, Watson SP, UK GAPP study group. Utility of the ISTH bleeding assessment tool in predicting platelet defects in participants with suspected inherited platelet function disorders. J Thromb Haemost 2013; 11(9): 1663–1668.
5. Shattil SJ, Cunningham M, Hoxie JA. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood 1987; 70(1): 307–315.
6. Lindahl TL, Festin R, Larsson A. Studies of fibrinogen binding to platelets by flow cytometry: an improved method for studies of platelet activation. Thromb Haemost 1992; 68(2): 221–225.
7. Michelson A. Flow cytometry: a clinical test of platelet function. Blood 1996; 87: 4925–4936.
8. Frelinger AL, 3rd, Grace RF, Gerrits AJ, Berny-Lang MA, Brown T, Carmichael SL, Neufeld EJ, Michelson AD. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood 2015; 126(7): 873–880.
9. Ramström S, Södergren AL, Tynngård N, Lindahl TL. Platelet function determined by flow cytometry: new perspectives? Semin Thromb Hemost 2016; 42(3): 268–281.
10. Rubak P, Nissen PH, Kristensen SD, Hvas A-M. Investigation of platelet function and platelet disorders using flow cytometry. Platelets 2015; 27(1): 66–74.
11. Boknäs N, Ramström S, Faxälv L, Lindahl TL. Flow cytometry-based platelet function testing is predictive of symptom burden in a cohort of bleeders. Platelets 2017; doi: https://doi.org/10.1080/09537104.2017.1349305
12. Huskens D, Sang Y, Konings J, van der Vorm L, de Laat B, Kelchtermans H, Roest M. Standardization and reference ranges for whole blood platelet function measurements using a flow cytometric platelet activation test. PLoS One 2018; 13(2): 1–16.
13. Andres O, Henning K, Strauß G, Pflug A, Manukjan G, Schulze H. Diagnosis of platelet function disorders: a standardized, rational, and modular flow cytometric approach. Platelets 2017; doi: 10.1080/09537104.2017.1386297.
The authors
Niklas Boknäs*1,2 MD, PhD; Sofia Ramström3,4 PhD; Tomas Lindahl3 MD, PhD
1Department of Hematology and Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden
2Australian Centre for Blood Diseases, Monash University, Melbourne, Australia
3Department of Clinical Chemistry and Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden
4School of Medical Sciences, Örebro University, Örebro, Sweden
*Corresponding author
E-mail: niklas.boknas@gmail.com