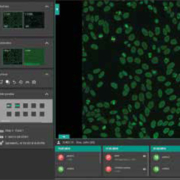
Euroimmun releases flexible laboratory management software
The EUROLabOffice 4.0 system provides a flexible central interface in the diagnostic laboratory, ensuring efficient workflow for many different applications and requirements. The software encompasses indirect immunofluorescence, ELISA, chemiluminescence, immunoblot and microarray analyses in autoimmune, infection, allergy and molecular genetic diagnostics. Continuous data exchange with the LIS and instruments ensures that all processes run smoothly, making the system suitable for random access applications. The software is simple and intuitive to operate, with no unnecessary manual steps, thus saving time and reducing workload for laboratory staff. The clear user interface makes it ideal for all workplaces, including the laboratory, microscopy room and office. Predefinable workflows and automatically created protocols ensure efficient organisation of the daily workload and thus faster generation of results. The software incorporates fully automated evaluation of indirect immunofluorescence results, including pattern recognition for antinuclear antibodies (ANA, based on the ICAP consensus) and anti-neutrophil cytoplasmic antibodies (ANCA). For Crithidiae luciliae (CLIFT), cell-based assays (e.g. anti-neuronal antibodies) and antigen dots (EUROPLUS), the system offers clear positive/negative classification. Furthermore, the program provides titer estimations with confidence values. The software consolidates all results into one report per patient, which includes the patient’s complete history and all images. Different user levels provide a hierarchical review of results, thus increasing security. After the verification procedure, result reports can be signed electronically and forwarded at the click of the mouse or exported into an existing LIS. Patient data, customer details and article information are all administered and archived by the system, ensuring a streamlined laboratory routine.
For more information, visit: www.euroimmun.com
Supplier: [No title]
Website: