Evaluation of protein induced by vitamin K absence II as a novel tumour marker in pancreatic cancer
Pancreatic cancer is a highly lethal malignancy: as its poor prognosis is also related to a difficulty of early diagnosis, a reliable and easily detectable biomarker is urgently needed. This article aims to describe a new serum tumour marker for pancreatic cancer, protein induced by vitamin K absence II.
by Dr Sara Tartaglione, Prof. Antonio Angeloni and Prof. Emanuela Anastasi
Pancreatic cancer
Pancreatic cancer (PC) is the 11th most common cancer in the world counting 458.918 new cases and causing 432.242 deaths (4.5.% of all deaths caused by cancer) in 2018 [1].
There are two main causes of PC: (i) pancreatic ductal adeno-carcinoma (PDAC), which occurs in the exocrine glands of the pancreas and is by far the most common (85.% of PC), and (ii) pancreatic neuroendocrine tumours, which arise in pancreatic endocrine tissue, are less common (<5.%) and have a better prognosis owing to its quite specific-symptoms. On the contrary, at its early stages, PDAC is usually clinically silent, and even upon progression of the neoplasm the symptoms are few and non-specific, such as weight loss, abdominal pain, jaundice, dyspepsia, light-colored stools and fatigue. PC has a very poor prognosis: the diverse and delayed symptoms of this disease are in part why 80.% of PDAC patients are usually diagnosed at an advanced or metastatic stage of disease, when the 5-year survival is less than 10.% [2]. At these stages, in fact, only 10–20.% of PDAC patients are treatable with surgery. When PC is identified in stage I, patients have a 5-year survival rate of 25.%, having a chance of successful resection and possible cure [3]. Surgery, chemotherapy and radiotherapy are traditionally used to extend survival and/or relieve the patients’ symptoms but for advanced stage PC cases, there is still no definitive treatment pathway. Indeed, in spite of recent progress in the clinical management of PC, its overall survival rate has not raised during the last two decades [4]. Therefore, PC still represents a major challenge for both research studies and clinical management.
Challenges in PC detection
Detection of PC at the early stages remains a great challenge because of a lack of specific detection tests. To date, there are several imaging tools available, such as abdominal ultrasonography, multidetector computed tomography (the standard for diagnosis), magnetic resonance imaging and endoscopic ultrasound-guided fine-needle aspiration for cytological diagnosis (which has a sensitivity reported to be about 80.%). Each of these techniques has its own advantages and disadvantages but are all susceptive to operator variability and the change in use of various diagnostic modalities differs between developed and undeveloped countries. Since the available diagnostic tests are non-specific and may miss patients with early-stage disease, many studies and clinical trials have sought to identify an inexpensive and minimally invasive biomarker with high sensitivity and specificity for PC to improve early diagnosis and subsequent treatment [2]. Many investigations have been conducted to find an appropriate serum biomarker. At present several tumour markers, such as CA 19-9, CA242, carcino-embryonic antigen (CEA), have been proposed for PC management, even if their benefits remain unclear: although sensitivity is increased, specificity is often sub-optimal [5]. The recommendations in existing clinical practice guidelines on early diagnosis of PC are inconsistent: most of them endorse measuring serum CA 19-9 as a complementary test, but also stated that it is not useful for diagnosing early pancreatic cancer or for screening. For these reasons an abundance of research in recent years has focused on identifying biomarkers for PC and there is a constant ongoing effort to identify additional ones.
A novel serum biomarker: protein induced by vitamin K absence II
In a recent preliminary study investigating a novel serum biomarker in PC, we reported for the first time that protein induced by vitamin K absence II (PIVKA-II) is significantly increased in a cohort of Italian PC patients. PIVKA-II, also known as des-gamma-carboxy prothrombin, is released by the liver in situations of vitamin K insufficiency or as consequence of an acquired defect in the post-translational carboxylation of the prothrombin precursor in cancer cells [6]. PIVKA-II is an abnormal prothrombin containing some glutamic acid (Glu) residues. When prothrombin is generated by liver cells under conditions of reduced vitamin K or in the presence of a vitamin K antagonist, the Glu residue at the N-terminal of the prothrombin precursor is not completely converted to carboxyglutamic acid (Gla) by gamma-carboxylase. Until the 1980s, the resulting protein, PIVKA-II, was mainly used as an indicator of blood coagulability and currently it is a useful tool for detecting subclinical vitamin K deficiency, as it increases before the prothrombin time is affected [7]. Since Liebman et al. in 1984 reported detection of a high rate of serum PIVKA-II in hepatocellular carcinoma (HCC) patients, this biomarker has been widely used for this neoplasm and it presently represents an important tool in its diagnosis and prognosis [8 and references therein]. Additionally, a rise in PIVKA-II above normal limits has been recently reported in literature (mainly from Japan) not only in HCC but also in other gastrointestinal malignancies, including PC. The mechanism of PIVKA-II production in non-HCC tumours is currently unknown. Hepatoid differentiation of tumours has been speculated as one of the mechanisms of PIVKA-II production. However, it has been showed that 10 of 23 reported cases of PIVKA-II-producing tumours (43.4.%) did not have hepatoid differentiation. It has been demonstrated that PIVKA-II-producing tumours show a high rate of liver metastasis and poor prognosis [9]. It is widely assumed that the pancreas and the liver retain a latent capability to transdifferentiate into the other tissue because they originate from the primitive foregut of the embryo [10]. Therefore, PIVKA-II expression, which is characteristic of HCC, can reasonably be present in PC, even if the mechanism of PIVKA-II pancreatic production is still unknown. Recently there is rising attention on the relationship between vitamin K and malignancy: several large population studies have established a relationship between vitamin K intake and cancer mortality. A number of studies have shown a cytotoxicity of vitamin K towards cancer cells. Various mechanisms, responsible for cell growth arrest and suppression of proliferation by vitamin K, have been described, however, almost all of them are focused on modulation of redox balance and induction of oxidative stress in cancer cells due to the quinone structure of vitamin K. In vitro and in vivo studies have suggested anti-carcinogenic effects exerted by vitamin K both directly (given its ability to suppress cancer growth, induce apoptosis and differentiation in cancer cells) and indirectly through post-translational activation of proteins such as PIVKA-II [11]. Multiple studies have recently evaluated the role of vitamin K against PC cell oncogenesis and it has been lately confirmed that apoptosis is mainly involved in vitamin K -induced pancreatic PC cell death. It has been demonstrated that vitamin K causes apoptosis of pancreatic cancer cells through either caspase-dependent mechanisms and induction of ERK phosphorylation, or via intracellular calcium, reactive oxygen species, and wild-type p53, respectively [12].
Pilot study
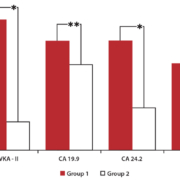
According to all these recent findings, our study was the first to investigate the role of PIVKA-II as a new biomarker for PC [13]. In this aim, a total of 46 Caucasian patients, 26 with PC (Group 1) and 20 with benign pancreatic diseases (Group 2), matched for age and sex, were enrolled from subjects attending the laboratory of Tumour Markers of the Policlinico Umberto I, Sapienza University of Rome. We aimed to evaluate PIVKA-II performance in comparison to established PC biomarkers CA 19-9, CEA and CA242. PIVKA-II and CEA serum levels were measured on LUMIPULSE G1200 (Fujirebio-Europe), an assay system based on chemiluminescent enzyme immunoassay (CLEIA) technology by a two-step sandwich in immunoreaction cartridges [14]. Serum CA 19-9 was measured by a RIA method ELSA (CisBio Bioassays), a solid-phase two-step sandwich immunometric assay, whereas CA242 levels were determined by an enzyme immunoassay (EIA) technique (Fujirebio Diagnostics AB), a solid-phase, non-competitive immunoassay. All assays were performed according to the manufacturers’ instructions and cut-offs of normality were considered as 6|ng/ml, 37|U/ml, 16|U/ml, and 48|mAU/ml, respectively for CEA, CA 19-9, CA242 and PIVKA-II. In PC patients, we observed high levels of PIVKA-II in the highest percentage of cases compared to other biomarkers, while in patients with benign pancreatic diseases PIVKA-II was increased in the smallest percentage of subjects compared to CA 19-9, CA242 and CEA. All markers showed statistically significant differences between Group 1 and Group 2 patients (Fig. 1). Diagnostic performance of the markers in discriminating malignant from benign gynecologic conditions was verified using receiver operator characteristic (ROC) curve analysis. The PIVKA-II ROC curve analysis showed the best specificity and sensitivity in comparison with CEA, CA 19-9 or CA242 (Table 1). This pilot study showed that PIVKA-II is significantly higher in PC than in benign pancreatic disease: as a serum tumour marker it demonstrated a rather good diagnostic performance compared to traditional PC biomarkers like CA 19-9, CEA and CA242, being less prone to elevation in patients with non-neoplastic pancreatic diseases.
Summary
Our results are promising as the discovery of a biomarker that would facilitate earlier identification of PC would greatly affect patient management and prognosis. Moreover, it has been reported that a combination of serum biomarkers increases specificity and sensitivity of the individual test: in this aim the detection of a biomarker to complement others in diagnostic accuracy would be of real importance for facilitating PC detection [15]. In conclusion, our study suggested that including serum PIVKA-II measurement in the diagnostic work-up for PC could be considered a valuable additional tool in clinical practice.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 107: 843–847.
2. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10(1): 10–27.
3. Thomas C. Risk factors, biomarker and imaging techniques used for pancreatic cancer screening. Chin Clin Oncol 2017; 6: 61.
4. Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, Li J, Huang K. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Prac 2016; 2016: 8962321.
5. Herreros-Villanueva M, Bujanda L. Non-invasive biomarkers in pancreatic cancer diagnosis: what we need versus what we have. Ann Transl Med 2016; 4(7): 134.
6. Dahlberg S, Nilsson CU, Kander T, Schött U. Detection of subclinical vitamin K deficiency in neurosurgery with PIVKA-II. Scand J Clin Lab Invest 2017; 77: 267–274.
7. Dauti F, Hjaltalin Jonsson M, Hillarp A, Bentzer P, Schött U. Perioperative changes in PIVKA-II. Scand J Clin Lab Invest 2015; 75(7): 562–567.
8. Viggiani V, Palombi S, Gennarini G, D’Ettorre G, De Vito C, Angeloni A, Frati L, Anastasi E Protein induced by vitamin K absence or antagonist-II (PIVKA-II) specifically increased in Italian hepatocellular carcinoma patients. Scand J Gastroenterol. 2016; 51: 1257–1262.
9. Kurohama H, Mihara Y, Izumi Y, Kamata M, Nagashima S, Komori A, Matsuoka Y, Ueki N, Nakashima M, Ito M. Protein induced by vitamin K absence or antagonist II (PIVKA-II) producing large cell neuroendocrine carcinoma (LCNEC) of lung with multiple liver metastases: a case report. Pathol Int 2017; 67(2): 105–109.
10. Gordillo M, Evans T, Gouon-Evans V. Orchestrating liver development. Development 2015; 142: 2094–108.
11. Dahlberg S, Ede J, Schött U. Vitamin K and cancer. Scand J Clin Lab Invest 2017; 77: 555–567.
12. Davis-Yadley AH, Malafa MP. Vitamins in pancreatic cancer: a review of underlying mechanisms and future applications. Adv Nutr 2015; 6: 774–802.
13. Tartaglione S, Pecorella I, Zarrillo SR, Granato T, Viggiani V, Manganaro L, Marchese C, Angeloni A, Anastasi E. Protein Induced by Vitamin K Absence II (PIVKA-II) as a potential serological biomarker in pancreatic cancer: a pilot study. Biochem Med (Zagreb) 2019; 29(2): 020707.
14. Falzarano R, Viggiani V, Michienzi S, Longo F, Tudini S, Frati L, Anastasi E. Evaluation of a CLEIA automated assay system for the detection of a panel of tumor markers. Tumour Biol 2013; 34(5): 3093–3100.
15. Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA 19.9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med 2015; 8(7): 11683–11691.
The authors
Sara Tartaglione*1 MD, Antonio Angeloni2 BS, Prof. Emanuela Anastasi1 BS
1 Department of Molecular Medicine, Policlinico Umberto I, University of Rome “Sapienza”, Rome, Italy
2 Department of Experimental Medicine, Policlinico Umberto I, University of Rome “Sapienza”, Rome, Italy
*Corresponding author
E-mail: sara.tartaglione@uniroma1.it