Fungal antigen detection as an aid for diagnosis of invasive fungal infections
Immunocompromised hosts are at special risk for invasive fungal infections (IFI). Traditional methods of diagnosis (e.g. culture and molecular methods) largely suffer from poor sensitivity, thus limiting their clinical utility. Enzyme immunoassay-based detection of fungal antigens represents an attractive, supplementary method for IFI identification and is the focus of this review.
by Phillip R. Heaton and Elitza S. Theel
Background
Invasive fungal infections (IFI) are a significant cause of morbidity and mortality in patients with hematologic malignancies and in hematopoietic stem cell transplant (HSCT) recipients. Although Aspergillus species and Candida albicans are among the most common agents of IFI, an increasing incidence of IFI due to other filamentous fungi (e.g. Fusarium, Zygomycetes) and non-albicans Candida (e.g. C. tropicalis, C. krusei, C. glabrata) has been reported. Currently, IFI diagnosis is based on clinical evaluation, radiologic imaging (both of which may lack clinical specificity) and culture-based laboratory findings. Unfortunately, culture of the aforementioned fungi from bronchoalveolar lavage fluid (BAL) and blood, the most commonly collected specimens in suspected IFI cases, suffers from poor sensitivity: only 45 to 60% of BAL specimens and up to 50% of blood cultures yield fungal growth [1]. Additionally, as some fungi are common in the environment (e.g. Aspergillus), providers must determine whether growth from BAL cultures is indicative of invasive disease or colonization of the respiratory tract. Finally, procedures to collect alternative specimens, including lung tissue, are often contra-indicated due to the critical state of the patient. These limitations have led to a clinical need for alternative methods to identify IFI – techniques independent of culture, which are both sensitive and specific. This demand has driven the development of novel assays to detect fungal biomarkers including the Aspergillus galactomannan (GM) antigen, the (1→3) β-D-glucan (BDG) polysaccharide common to many fungi, and the Candida mannan antigen (Mn-A). This brief review will discuss the clinical utility, advantages and limitations of GM, BDG and Mn-A detection assays in patients at risk for IFI.
Detection of the Aspergillus galactomannan antigen
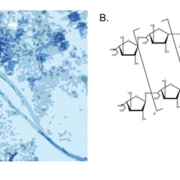
Galactomannan, composed of a mannan core and highly immunogenic galactofuranosyl side chains, is a dominant cell wall component present in the majority of clinically relevant Aspergillus species and is released during hyphal growth into surrounding tissue (Fig. 1). Currently, the Platelia Aspergillus antigen (Bio-Rad, Marnes-la-Coquette, France) enzyme immunoassay (EIA) is the only FDA approved assay for GM detection of in serum and BAL fluid, though other kits are also available (e.g. Pastorex kit, Sanofi Diagnostics, Pasteur, Marnes-La-Coquette, France). The Platelia EIA is a quantitative assay with GM levels ≥0.5 ng/mL considered as positive. The presence of GM in patient specimens can be used as an aid, alongside other clinical studies, to specifically detect invasive aspergillosis (IA), a potentially devastating condition encountered in 5–20% of HSCT patients [2]. The performance characteristics of the Platelia GM assay have been widely evaluated with overall favorable outcomes. Briefly, one study reported a clinical sensitivity and specificity of 94.4% and 98.8% respectively, from serum of HSCT patients with proven or probable IA [as defined by the European Organization for Research and Treatment of Cancer (EORTC) criteria], with similar positive and negative predictive values [3]. While a subsequent meta-analysis of GM studies found a significantly lower sensitivity in this patient population (58%), specificity remained comparable at 95% [4]. Notably, these results are in stark contrast to the sensitivity of this assay in other immunocompromised (ICH) patient populations, specifically in solid organ transplant (SOT) recipients, where sensitivity can be as low as 22–41% [4]. Additionally, while the kinetics of GM clearance are not yet well defined, serial testing and trending of GM levels following initiation of antifungal therapy has been shown to correlate well with patient outcome. Specifically, while persistently elevated GM levels were associated with treatment failure, a decrease of GM levels by ≥35% between baseline and week one of antifungal treatment was associated with clinical improvement [5].
Despite the advantage of rapid GM testing in serum, a readily available specimen source, and the potential to monitor response to therapy, a number of limitations affecting assay specificity have been described. First, false-positive GM reactions have been associated with prior (<12 hours) administration of piperacillin/tazobactam, a fungal-derived antibiotic [6]. Recently, however, Mikulska and colleagues demonstrated negligible GM levels in patients on piperacillin/tazobactam therapy, suggesting that modern day manufacturing practices may have improved antibiotic purity [7]. Nonspecific reactions have also been noted in patients with non-Aspergillus IFI, including Fusarium and Penicillium (an exceedingly rare agent of IFI which also expresses GM) species infections and in individuals with either graft versus host disease (GVHD) or a damaged intestinal wall through which GM from food products can translocate [8].
Detection of β-D-glucan, a pan-fungal biomarker
(1→3)-β-D-glucan (BDG) is an abundant cell wall polysaccharide found in most fungi with the exception of Cryptococcus species, the Zygomycetes and Blastomyces dermatitidis (Fig. 2). The most commonly used BDG detection method, the Fungitell assay (Associates of Cape Cod, East Falmouth, MA), is a quantitative EIA (values ≥60 pg/mL considered positive) which detects BDG in serum using a modified version of the Limulus (horseshoe crab) clotting cascade. As a pan-fungal biomarker, BDG detection in patients with hematologic malignancies and HSCT recipients has been associated with high clinical specificity (76–99%) and negative predictive values (87–96%) for the presence of proven or probable IFI [9]. Similar to the GM assay however, inclusion of other ICH groups (e.g. SOT patients) dramatically lowers the performance characteristics of this assay. Interestingly, regardless of the patient population, the associated clinical sensitivity and positive predictive value of the BDG assay are generally poor (range 38 – 80%), collectively indicating that a single, negative BDG result should not be used to exclude the diagnosis of IFI [9]. Serial BDG testing, however, can significantly improve the clinical sensitivity of this assay and trending BDG levels during antifungal therapy has some prognostic value with respect to treatment failure or response, particularly in patients with disseminated candidiasis [9, 10]. Furthermore, BDG was shown to be detectable in critically ill patients prior to development of clinical symptoms, radiological findings or culture positivity, suggesting that in patients at increased risk for IFI, the presence of BDG should warrant further evaluation to identify an infectious process [11]. Finally, in patients with Pneumocystis jirovecii pneumonia (PjP), for whom invasive BAL or biopsy procedures are often precluded due to safety concerns, the demonstration of elevated BDG levels has been associated with high clinical sensitivity (>95%) [12]. Though this data is encouraging, especially in light of the limited sensitivity of current diagnostic methods to detect P. jirovecii, due to the pan-fungal nature of BDG, a positive result cannot be used to diagnose PjP pneumonia; a negative BDG finding can, however, be used to potentially exclude P. jirovecii as the causative agent.
The greatest limitation of BDG assays is their poor specificity. Many studies have now documented the generation of false-positive results in patients who received or have been exposed to albumin, intravenous immunoglobulin, amoxicillin-clavulanic acid, gauze during surgery, or cellulose based filters during dialysis. Additionally, infection with certain bacterial agents, including Alcaligenes faecalis, can also lead to false-positive results. Therefore, providers using these assays must confidently exclude these confounding factors prior to interpreting BDG results.
Detection of the Candida mannan antigen
The Candida Mn-A is an oligomannan cell wall component which can be detected by multiple quantitative EIAs (Fig. 3). Currently, the Platelia Candida Ag Plus quantitative EIA (Bio-Rad) is most commonly used. A recent meta-analysis of 14 studies evaluating the utility of Mn-A detection found significant heterogeneity in clinical sensitivity for detection of invasive candidiasis (IC), which, interestingly, appeared to be species dependent. For example, among patients with disseminated C. albicans, C. glabrata or C. tropicalis, the sensitivities ranged from 58–70%, whereas for patients with invasive C. parapsilossis and C. krusei, sensitivity of the assay ranged between 25–30% [13]. Importantly, however, despite the low sensitivity of the assay, the majority of Mn-A positive patients were subsequently confirmed as culture positive, suggesting the utility of this assay as an early diagnostic marker in at risk patients. Notably, the specificity of this assay is high (>90%) with cross-reactivity reported in patients with Geotrichum or Fusarium species infections, both fairly uncommon [14]. Due to the described performance variability and the short duration of Mn-A circulation, many authors have suggested combination testing with anti-Mn antibodies (Platelia Candida Ab Plus, Bio-Rad), which are detectable in at risk patients >10 days prior to proven candidemia [15]. One study evaluated neutropenic patients using combination testing and found that Mn-A/anti-Mn outperformed traditional diagnostic methods (cultures, radiology, and histopathology) for detection of IC with a sensitivity and specificity of 89% and 84%, respectively [15]. Based on these and other studies, current ECIL-3 recommendations support using a combination of Mn-A and antibody testing as an aid to detect IC [13].
Conclusions
The diagnosis of IFI in ICHs remains a challenge, and despite the limited sensitivity and specificity of the various fungal antigen detection assays, in 2008 the EORTC included detection of GM and BDG as supportive evidence for proven or probable IFI in specific patient populations [3]. When used appropriately (i.e. serial testing of high risk patients) and by providers knowledgeable of the associated limitations, antigen detection can be crucial marker for the identification of IFI. Future advancement of IFI diagnostics lies in the molecular arena and real-time polymerase chain reaction (RT-PCR) assays to detect fungal nucleic acid.
References
1. Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005; 18: 44–69.
2. Tamma P. The Galactomannan antigen assay. Pediatr Infect Dis J. 2007; 26: 641-642 610.1097/INF.1090b1013e318070c318525.
3. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46: 1813–1821.
4. Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006; 42: 1417–1727.
5. Chai LY, Kullberg BJ, Johnson EM, Teerenstra S, Khin LW, Vonk AG, Maertens J, Lortholary O, Donnelly PJ, Schlamm HT, Troke PF, Netea MG, Herbrecht R. Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol. 2012; 50: 2330–2336.
6. Machetti M, Majabo MJ, Furfaro E, Solari N, Novelli A, Cafiero F, Viscoli C. Kinetics of galactomannan in surgical patients receiving perioperative piperacillin/tazobactam prophylaxis. J Antimicrob Chemother. 2006; 58: 806–810.
7. Mikulska M, Furfaro E, Del Bono V, Raiola AM, Ratto S, Bacigalupo A, Viscoli C. Piperacillin/tazobactam (TazocinTM) seems to be no longer responsible for false-positive results of the galactomannan assay.
J Antimicrob Chemother. 2012; 67: 1746–1748.
8. Mennink-Kersten MASH, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004; 4: 349–357.
9. Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O. Beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis. 2012; 54: 633–643.
10. Jaijakul S, Vazquez JA, Swanson RN, Ostrosky-Zeichner L. (1,3)-β-D-Glucan as a prognostic marker of treatment response in invasive candidiasis. Clin Infect Dis. 2012; 55: 521–526.
11. Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004; 39: 199–205.
12. Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013; 19: 39–49.
13. Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012; 47: 846–854.
14. Rimek D, Singh J, Kappe R. Cross-reactivity of the PLATELIA CANDIDA antigen detection enzyme immunoassay with fungal antigen extracts. J Clin Microbiol. 2003; 41: 3395–3398.
15. Prella M, Bille J, Pugnale M, Duvoisin B, Cavassini M, Calandra T, Marchetti O. Early diagnosis of invasive candidiasis with mannan antigenemia and antimannan antibodies. Diagn Microbiol Infect Dis. 2005; 51: 95–101.
The authors
Phillip R. Heaton PhD and Elitza S. Theel PhD*
Division of Clinical Microbiology,
Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester,
Minnesota, USA
*Corresponding author
E-mail: theel.elitza@mayo.edu