MTHFR, hyperhomocysteinemia, CAD and T2DM
Individuals with type 2 diabetes mellitus (T2DM) are at increased risk of coronary artery disease (CAD). The C677T mutation of the methylenetetrahydrofolate reductase (MTHFR) gene is associated with elevated plasma levels of homocysteine. The association of the MTHFR gene and the level of homocysteine with development of CAD has been studied in various population groups, including patients with T2DM, but the results have been variable. In practice, plasma homocysteine may be ordered as part of a screen for people with CAD or stroke, or who are at high risk for CAD or stroke but no other known risk factors. Testing of C677T polymorphism with or without elevated homocysteine is not recommended and has no clinical utility.
by Prof. Bakri Saeed and Dr Nisreen Mohammed
Type 2 diabetes mellitus and coronary artery disease
Type 2 diabetes mellitus (T2DM) is a major health problem throughout the world. It is a polygenic and multifactorial disease that is a major risk factor for cardiovascular disease. Cardiovascular disease (CVD) comprises coronary artery disease (CAD), also referred to as coronary heart disease (CHD), or ischemic heart disease (IHD), and cerebrovascular disease.
CAD due to atherosclerosis is a cause of significant morbidity and mortality, and is the leading cause of death worldwide. There are several risk factors for CAD. The well-stablished risk factors for CAD include diabetes mellitus, hypertension, smoking and dyslipidemia. There is growing interest in emerging risk factors for improved understanding of the mechanisms that underline cardiovascular disorders and CAD.
T2DM increases the risk for CAD by 2–4-fold compared to people without diabetes. CVD accounts for about 70% of deaths in people with diabetes. Identification and management of risk factors for CAD is an important aspect of management of diabetes mellitus.
Hyperhomocysteinemia and MTHFR polymorphism
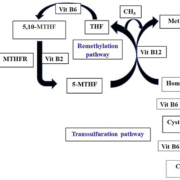
Homocysteine is a sulfur-containing amino acid formed from demethylation of methionine. Methionine is the precursor to S-adenosyl methionine (SAMe) and is one of the essential amino acids. SAMe is a major methyl donor and is involved in numerous biological reactions. Homocysteine is metabolized by either remethylation to methionine or transsulfuration to cystathionine. The former reaction is catalysed by the vitamin B12-dependent methionine synthase. The latter reaction is catalysed by the enzyme cystathionine beta-synthase, which requires vitamin B6.
The methyl donor in the remethylation of homocysteine to methionine is 5-methyltetrahydrofolate. The 5,10-methylene-tetrahydrofolate reductase (MTHFR) enzyme catalyses the reduction of 5,10-methylene-tetrahydrofolate to 5-methyltetrahydrofolate. The enzyme requires B2 (riboflavin) as a cofactor (Fig. 1).
Therefore, hyperhomocysteinemia can result from reduced activity of the enzymes involved in homocysteine metabolism or from deficiency of the vitamins which are needed as cofactors in homocysteine metabolic reactions: folate, vitamin B6 and vitamin B12.
Several mutations in the MTHFR gene have been identified and some of them affect the activity of the enzyme. The commonest MTHFR gene mutation is a cytosine-to-thymidine substitution at nucleotide 677 (C677T), which changes alanine into valine, resulting in a thermolabile enzyme with impaired enzymatic activity and leading to hyperhomocysteinemia.
There are two copies of each gene. Therefore, an individual can be homozygous for the mutated gene or can be heterozygous, having one copy of the C677T variant and one normal copy. The C677T homozygous variant enzyme is thermolabile and demonstrates 70% reduced enzyme activity in vitro. The heterozygous C677T MTHFR enzyme has 35% reduced activity in vitro.
Worldwide, the frequency of MTHFR gene mutations varies among racial and ethnic groups, in Africa MTHFR gene polymorphism is markedly low (below 10%) for the C677T allele. In the European and Asian population, estimates of 18.6% and 20.8% were reported [1].
Association with CAD
In recent years hyperhomocysteinemia has been implicated as a risk factor for CAD, independent of other known risk factors. The primary mechanism by which homocysteine promotes atherosclerosis is by impairing endothelial function, which initiates the chain of events resulting in atherosclerotic plaque formation.
Numerous studies looked into the possible association between MTHFR genotypes and plasma homocysteine levels and the incidence of different MTHFR genotypes and hyperhomocysteinemia in CAD patients [2–5]. The results of these studies have been controversial. Several studies have shown the link between the MTHFR C677T gene polymorphism and the risk for CAD but many other studies failed to show association between MTHFR genotypes and plasma homocysteine levels and their role in CAD.
Previous studies in T2DM patients were also controversial. MTHFR polymorphism and hyperhomocysteinemia were shown to be predictors of cardiovascular events among diabetic patients [6, 7], whereas other studies failed to show a role for MTHFR polymorphic variants and homocysteine in increasing susceptibility to cardiovascular disease [8, 9].
Our study
We recently screened 226 consecutive patients with T2DM, <60 years of age, diagnosed according to WHO criteria. Of these, 113 had CAD confirmed by angiography and electrocardiography (ECG) and 113 had no evidence of CAD [10]. PCR and restriction fragment length polymorphism (RFLP) using Hinf1 restriction enzyme were used to determine MTHFR genotypes.
In our study, the T allele had a significant effect on homocysteine level (P value <0.05) and showed strong association with CAD among T2DM patients (odds ratio 6.2, P <0.0001).
Our study indicates that the C677T polymorphism of the MTHFR gene is associated with hyperhomocysteinemia, and the two are independently associated with the presence of CAD in patients with T2DM.
Reasons for controversy
The outcome of these numerous studies and meta-analysis remained contradictory. There was no agreement on the association between MTHFR genotypes and plasma homocysteine levels or the incidence of different MTHFR genotypes and hyperhomocysteinemia in CAD patients.
Plasma homocysteine levels are dependent on interacting nutritional and genetic factors. Some studies suggested that people homozygous for MTHFR C667T polymorphism tend to have hyperhomocysteinemia in the context of low folic acid levels. Supplementation with the vitamins involved in homocysteine metabolism was found to lower plasma homocysteine levels.
Therefore, geographic heterogeneity, nutritional and environmental factors could affect the relationship between MTHFR genotypes and CVD risk in different populations.
Practical points
Homocysteine may be ordered as part of a screen for people with or at high risk of CAD or stroke, especially if there is family history of CAD or stroke but no other known risk factors, such as diabetes, smoking, hypertension, or dyslipidemia. Routine screening of homocysteine, like that of cholesterol, has not been recommended.
Plasma homocysteine concentration may be elevated in B12 and folate deficiency and its measurement has been suggested to give an early indicator of deficiency.
In new-born testing, greatly increased concentrations of homocysteine in the urine and blood suggests a diagnosis of homocystinuria and indicates the need for confirmation of the cause of raised levels.
Most laboratories report normal homocysteine levels in the blood between 5 and 15 µmol/L. Any measurement above 15 µmol/L is considered high.
However, it should be noted that normal levels will vary between ethnic groups and populations. Homocysteine levels increase with age, are lower in pregnancy and are influenced by drugs. These factors should be taken into consideration when interpreting results.
Testing of C677T polymorphism with or without elevated homocysteine is not recommended in patients with CAD or other diseases where MTHFR variants have been implicated, such as thrombophilia or recurrent pregnancy loss.
References
1. Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet 1998; 62: 1258–1260.
2. Chehadeh SWEH, Jelinek HF, Al Mahmeed WA, Tay GK, Odama UO, Elghazali GE, et al. Relationship between MTHFR C677T and A1298C gene polymorphisms and complications of type 2 diabetes mellitus in an Emirati population. Meta gene 2016; 9: 70–75.
3. Bickel C, Schnabel R, Zengin E, Lubos E, Rupprecht H, Lackner K, et al. Homocysteine concentration in coronary artery disease: Influence of three common single nucleotide polymorphisms. Nutr Metab Cardiovascular Dis 2017; 27(2): 168–175.
4. Yilmaz H, Isbir S, Agachan B, Ergen A, Farsak B, Isbir T. C677T mutation of methylenetetrahydrofolate reductase gene and serum homocysteine levels in Turkish patients with coronary artery disease. Cell Biochem Funct 2006; 24(1): 87–90.
5. Meisel C, Cascorbi I, Gerloff T, Stangl V, Laule M, Müller JM, et al. Identification of six methylenetetrahydrofolate reductase (MTHFR) genotypes resulting from common polymorphisms: impact on plasma homocysteine levels and development of coronary artery disease. Atherosclerosis 2001; 154(3): 651–658.
6. Lewis SJ, Ebrahim S, Smith GD. Meta-analysis of MTHFR 677C→T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ 2005; 331(7524): 1053–1058.
7. Bennouar N, Allami A, Azeddoug H, Bendris A, Laraqui A, El Jaffali A, et al. Thermolabile methylenetetrahydrofolate reductase C677T polymorphism and homocysteine are risk factors for coronary artery disease in Moroccan population. J Biomed Biotechnol 2007(1); 80687.
8. Bahadır A, Eroz R, Türker Y. Does the MTHFR C677T gene polymorphism indicate cardiovascular disease risk in type 2 diabetes mellitus patients? Anatolian J Cardiol 2015; 15(7): 524–530.
9. Rahimi Z, Nomani H, Mozafari H, Vaisi-Raygani A, Madani H, Malek-Khosravi S, et al. Factor V G1691A, prothrombin G20210A and methylenetetrahydrofolate reductase polymorphism C677T are not associated with coronary artery disease and type 2 diabetes mellitus in western Iran. Blood Coagul Fibrinolysis 2009; 20(4): 252–256.
10. Mohammed NO, Ali IA, Elamin BK and Saeed BO. The association of methylenetetrahydrofolate reductase gene polymorphism and hyperhomocysteinaemia with coronary artery disease in Sudanese patients with type 2 diabetes. Poster at Focus 2017, Association of Clinical Biochemistry annual meeting.
The authors
Bakri Osman Saeed*1 PhD, MD, FRCPath, FRCP; Nisreen Osman Mohamed2 PhD
1Faculty of Medicine, Sudan International University, Khartoum, Sudan
2Ahfad Centre for Science and Technology, Ahfad University for Women, Khartoum, Sudan
*Corresponding author
E-mail: saeedbakri@hotmail.com