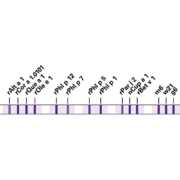
Multiparameter test for differential diagnosis of pollen allergies in Southern Europe
Specific IgE antibodies against inhalation allergens that commonly occur in Southern Europe can be detected and differentiated using the new multiparameter immunoblot EUROLINE DPA-Dx Pollen Southern Europe 1 based on defined partial allergens (DPA, allergen components). The detailed profile identifies the precise allergy-causing proteins, distinguishing primary sensitizations from cross reactions and thus aiding therapy decision-making. The test is based on a unique combination of species-specific marker molecules, cross-reactive components and whole extracts, encompassing tree pollens (birch, olive, cypress, hazel, oak), grass pollens (timothy grass, wall pellitory) and the fungus Alternaria alternata. The native extracts provide a general screening for IgE antibodies against the respective pollen/fungus. Combination of the Southern European species-specific marker molecules (e.g. nCup a 1, rOle e 1 and rPar j 2) together with common panallergens (e.g. PR-10 proteins and profilin) enables precise discrimination between a putative primary sensitization and a persistent cross reaction. Positive reactions with species-specific markers are an indication for specific immunotherapy. Positive reactions with panallergens on the other hand indicate cross reactions. Cross-reactive molecules are prevalent among different types of plants, which often results in a high sensitization rate. An insufficient differentiation between primary sensitization and cross reactions often results in a less effective desensitization during specific immunotherapy. The test also includes a band of cross-reactive carbohydrate determinants (CCD), which aids the differentiation of clinically relevant reactions from unspecific anti-CCD reactions and thus the interpretation of results. With this in-depth profile, true sensitizations can be reliably identified, enabling targeted selection of the most suitable specific immunotherapy and improved prognosis of the therapy success. The test is easy to perform and requires only a small sample volume. The high analytical and clinical value of the profile has been verified in an independent study (publications in preparation). A further large-scale international multicentre study is currently in progress (www.ait2020.com). Automated processing and evaluation are available for the assay.
The new profile is part of the established EUROLINE Allergy range, which encompasses a comprehensive portfolio of component- and extract-based profiles for the diagnosis of food, inhalation and insect venom allergies.