Novel biomarkers in malignant pleural mesothelioma
Mesothelioma is a fatal cancer of mesothelial cells caused by previous asbestos exposure. Numerous biomarkers have been tested for their ability to diagnose or monitor pleural mesothelioma, but none are in routine clinical practice. This article aims to briefly outline the literature to date and future research directions.
by Dr David T. Arnold and Prof. Nick A. Maskell
Introduction
Mesothelioma is a cancer of mesothelial cells that carries a very poor prognosis. It is almost exclusively caused by previous inhalation of asbestos fibres, which is usually through industrial employments (ship building, lagging, railway work, etc) or from working on pre-existing asbestos products (plumbing, carpentry, etc). Given there is a 40-year mean latency from exposure to presentation, the European incidence of mesothelioma is expected to rise until around 2020, in keeping with the use and subsequent banning of asbestos in the 1980s [1]. However, given ongoing unregulated use of asbestos in China, India and Russia, cases of mesothelioma will continue to occur worldwide.
Mesothelioma can occur in the pleural cavity and peritoneum (with a 4 : 1 ratio) and more rarely in the pericardium and tunica vaginalis. Malignant pleural mesothelioma (MPM) is the most common with around 2500 new cases in the UK every year and will form the basis of the rest of this review [2].
Survival from MPM is dependent on histological subtype, of which there are four: epithelioid, sarcomatoid, biphasic and desmoplastic. Epithelioid accounts for 70 % of the overall cases and has the best prognosis, with a median survival of 13–14 months. Sarcomatoid has the worst prognosis, at 4 months, and is usually felt by clinicians to be not amenable to therapy.
Presentation
The main symptoms from MPM are shortness of breath, cough and chest pain. Given that it is a highly metabolically active tumour, patients can also develop systemic symptoms of fevers/sweats, fatigue and weight loss, indicating a more advanced stage. Around 90 % of individuals with MPM present with a pleural effusion (fluid collection around the lung), and any male with a history of asbestos exposure and a unilateral effusion has a 60 % of having malignancy [3]. MPM is highly locally invasive, which can cause chest pain, but rarely metastasises unless the pleura is disrupted by diagnostic or therapeutic procedures, which can cause tract metastases.
Diagnosis and imaging
Patients who present with a pleural effusion will invariably have cytological analysis of the fluid first. However, pleural fluid cytology alone is not usually sufficient to make a diagnosis of MPM [3]. If the patient is well enough then a biopsy is performed either radiologically, via medical thoracoscopy or surgically. These procedures are invasive, so there has been significant interest in additional diagnostic methods.
The mainstay of radiological investigations is computerised tomography (CT), with magnetic resonance imaging and positron emission tomography (PET) currently limited to the research setting. However, differentiating MPM from benign pleural thickening or other pleural malignancies is unreliable using CT alone [2]. Given the drawbacks in current cytopathological and radiological investigations for MPM there is a huge potential role for serum or pleural fluid biomarkers. Biomarkers would allow earlier detection of malignancy in at-risk groups (e.g. the asbestos exposed), reduce the need for invasive biopsies and speed the diagnostic pathway to treatment.
Treatment and monitoring
Sadly, due to the highly invasive nature of MPM, treatment is often palliative from diagnosis. The current standard of care is based on the results of a non-placebo-controlled trial from 2003. Vogelzang and colleagues used a combination of pemetrexed (an anti-folate) and cisplatin (platinum-based agent) chemotherapy [4]. This combination adds a modest 2 months to overall survival, with a response rate of only 30 %. Treatment for MPM had not significantly advanced until the publication of the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS) trial in 2015, which showed a 2-month survival advantage when bevacizumab [an anti-vascular endothelial growth factor (VEGF) immunotherapy] was added to standard chemotherapy [5].
The role of surgery for MPM is highly controversial, with significant variation in operative rates internationally. This controversy exists because there are no randomised trials of radical surgical intervention against best medical therapy. Large case series of patients with positive surgical outcomes exist, but they are often highly selective of younger patients with good performance status.
Both chemotherapeutic and surgical management would benefit from a greater ability to prognosticate patients at baseline and assess response to treatment. Currently, serial CT scanning is the gold standard of disease monitoring in MPM. Similar to other malignancies an attempt to measure change in tumour is made using the RECIST criteria. However, unlike other malignancies, MPM grows as a rind around the chest wall so volume measurement is difficult. A modified RECIST criteria has been developed, but is time intensive, and, with the added complications of pleural fluid and plaques, is rarely used outside the research setting. Other radiological methods to monitor disease have been examined, with a recognition that biomarkers would be ideal as a method of monitoring disease in a non-invasive manner [6].
Mesothelin
Soluble mesothelin (SM) is a 40 kDa glycoprotein over-expressed by the epithelioid component of malignant mesothelial cells. Its exact biological role remains uncertain. Discovered in the serum of patients with ovarian cancer, it was subsequently found in serum, pleural fluid and urine of patients with MPM. See Figure 1 in the open access article by Hassan et al. for a schematic showing the maturation and structure of mesothelin [7].
There has been considerable research attention on its utility in diagnosis or monitoring MPM. The majority of these studies have used a commercial platform, the Mesomark® ELISA. Unfortunately, despite some positive initial signals, a meta-analysis by Cui and colleagues in 2014 demonstrated that the overall sensitivity for detecting MPM was 0.61 in serum and 0.79 in pleural fluid [8]. The level of SM rises with increased epithelioid disease bulk, and, therefore, can be low in early-stage disease and may never rise in sarcomatoid or desmoplastic subtypes. For a diagnosis with such profound consequences for the individual, as well as medico-legal implications, this inability to reliably detect MPM has limited its widespread diagnostic use.
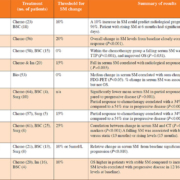
More recent research has focused on its ability to monitor MPM during treatment or follow-up (Table 1).
Although there is considerable heterogeneity between these 10 studies in terms of primary outcome measures, each has demonstrated that a rising SM is correlated with clinical or radiological disease progression. A falling SM following chemotherapy or surgery was strongly indicative of treatment response. The future of SM in disease monitoring depends on the results of currently recruiting prospective trials.
Fibulin-3
Fibulin-3 is a glycoprotein that promotes tumour growth and invasion through the phosphorylation of epidermal growth factor. In 2012, the New England Journal of Medicine published the results of a landmark study which reported that serum fibulin-3 had a 100 % sensitivity for detecting early-stage MPM [9]. Unfortunately, several follow-up studies using the same commercial ELISA have been unable to replicate these results. Ren and colleagues published a meta-analysis of eight studies which found the sensitivity to be around 87 % with a specificity of 89 % [10].
Osteopontin
Osteopontin is over-expressed in several malignancies and several studies have focused on its prognostic abilities in MPM. Early studies used serum osteopontin, without appreciating the impact of its thrombin cleavage site on results. More recent studies have used more accurate plasma measurement and shown that osteopontin has no role in diagnosis or monitoring [11]. Interestingly, there is evidence that baseline osteopontin is a marker of poor prognosis, independent of histology, treatment modality or other biomarkers.
Vascular Endothelial Growth Factor (VEGF)
VEGF has been studied as a potential diagnostic or therapeutic target in MPM. Although baseline VEGF correlates with disease stage and survival it is not used in the clinical setting. However, following the publication of the MAPS trial (of the anti-VEGF immunotherapy bevacizumab) there has been a renewed focus on whether baseline VEGF can select responders from non-responders. These studies have been directed at pan-VEGF, as opposed to any specific isoform, and have not shown any definitive role to date.
Proteomic studies
A modern approach to biomarker discovery and validation is exemplified by the DIAPHRAGM study [12]. Tsim and colleagues took the results of a promising 13-protein diagnostic panel developed and internally validated by Ostroff [12]. The internal validation cohort had reported an area under curve (AUC) for detecting MPM of 0.95 (38 patients with MPM). A flaw of previous biomarker validation is that follow-up work is performed on small retrospective cohorts. It often takes several years or decades before the true utility of the biomarker is established. The DIAPHRAGM study aims to quickly and definitively validate or reject the SOMAscan assay, alongside fibulin-3, in a prospective, powered and clinically relevant manner. The final results of the research are awaited, but regardless, this approach has set a standard for biomarker discovery and validation in MPM.
Summary
MPM is a highly aggressive cancer that is difficult to diagnose and monitor. The potential scope for biomarkers is huge. Serum SM has shown the most promise in monitoring disease. A biomarker that can reliably diagnose early-stage MPM remains elusive.
References
1. Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005; 353: 1591–1603.
2. Woolhouse I, Bishop L, Darlison L, de Fonseka D, Edey A, Edwards J, Faivre-Finn C, Fennell DA, Holmes S, et al. BTS guideline for the investigation and management of malignant pleural mesothelioma. BMJ Open Respir Res 2018; 5(1): e000266.
3. Arnold DT, De Fonseka D, Perry S, Morley A, Harvey JE, Medford A, Brett M, Maskell NA. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J 2018; 52(5): pii: 1801254.
4. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21(14): 2636–2644.
5. Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387(10026): 1405–1414.
6. Hooper CE, Lyburn ID, Searle J, Darby M, Hall T, Hall D, Morley A, White P, Rahman NM, et al. The South West Area Mesothelioma and Pemetrexed trial: a multicentre prospective observational study evaluating novel markers of chemotherapy response and prognostication. Br J Cancer 2015; 112(7): 1175–1182.
7. Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004; 10(12 Pt 1): 3937–3942 (http://clincancerres.aacrjournals.org/content/10/12/3937.long).
8. Cui A, Jin XG, Zhai K, Tong ZH, Shi HZ. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open 2014; 4(2): e004145.
9. Pass HI, Levin SM, Harbut MR, Melamed J, Chiriboga L, Donington J, Huflejt M, Carbone M, Chia D, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012; 367(15): 1417–1427.
10. Ren R, Yin P, Zhang Y, Zhou J, Zhou Y, Xu R, Lin H, Huang C. Diagnostic value of fibulin-3 for malignant pleural mesothelioma: A systematic review and meta-analysis. Oncotarget 2016; 7(51): 84851–84859.
11. Lin H, Shen YC, Long HY, Wang H, Luo ZY, Wei ZX, Hu SQ, Wen FQ. Performance of osteopontin in the diagnosis of malignant pleural mesothelioma: a meta-analysis. Int J Clin Exp Med 2014; 7(5): 1289–1296.
12. Tsim S, Kelly C, Alexander L, McCormick C, Thomson F, Woodward R, Foster JE, Stobo DB, Paul J et al. Diagnostic and Prognostic Biomarkers in the Rational Assessment of Mesothelioma (DIAPHRAGM) study: protocol of a prospective, multicentre, observational study. BMJ Open 2016; 6(11): e013324.
13. de Fonseka D, Arnold DT, Stadon L, Morley A, Keenan E, Darby M, Armstrong L, Virgo P1, Maskell NA. A prospective study to investigate the role of serial serum mesothelin in monitoring mesothelioma. BMC Cancer 2018; 18(1): 199.
14. Bonotti A, Simonini S, Pantani E, Giusti L, Donadio E, Mazzoni MR, Chella A, Marconi L, Ambrosino N, et al. Serum mesothelin, osteopontin and vimentin: useful markers for clinical monitoring of malignant pleural mesothelioma. Int J Biol Markers 2017; 32(1):e126–e131.
15. Hassan R, Sharon E, Thomas A, Zhang J, Ling A, Miettinen M, Kreitman RJ, Steinberg SM, Hollevoet K, Pastan I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014; 120: 3311–3319.
16. Nowak AK, Brown C, Millward MJ, Creaney J, Byrne MJ, Hughes B, Kremmidiotis G, Bibby DC, Leske AF, et al. A phase II clinical trial of the vascular disrupting agent BNC105P as second line chemotherapy for advanced malignant pleural mesothelioma. Lung Cancer 2013; 81: 422–427.
17. Franko A, Dolzan V, Kovac V, Arneric N, Dodic-Fikfak M. Soluble mesothelin-related peptides levels in patients with malignant mesothelioma. Dis Markers 2012; 32: 123–131.
18. Hollevoet K, Nackaerts K, Gosselin R, De Wever W, Bosquée L, De Vuyst P, Germonpré P, Kellen E, Legrand C, et al. Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J Thorac Oncol 2011; 6: 1930–1937.
19. Creaney J, Francis RJ, Dick IM, Musk AW, Robinson BW, Byrne MJ, Nowak AK. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res 2011; 17: 1181–1189.
20. Wheatley-Price P, Yang B, Patsios D, Patel D, Ma C, Xu W, Leighl N, Feld R, Cho BC, et al. Soluble mesothelin-related Peptide and osteopontin as markers of response in malignant mesothelioma. J Clin Oncol 2010; 28: 3316–3322.
21. Grigoriu BD, Chahine B, Vachani A, Gey T, Conti M, Sterman DH, Marchandise G, Porte H, Albelda SM, Scherpereel A. Kinetics of soluble mesothelin in patients with malignant pleural mesothelioma during treatment. Am J Respir Crit Care Med 2009; 179: 950–954.
The authors
David T. Arnold* MBBCh, BSc, MRCP and Nick A. Maskell BMedSci, BM, BS, FRCP, DM, FCCP
Academic Respiratory Unit, Learning and Research Building, Southmead Hospital, Bristol, UK
*Corresponding author
E-mail: arnold.dta@gmail.com