POCT in hospitals: the role of the clinical laboratory department – an Israeli hospital experience
Point-of-care testing (POCT) is becoming an important part of laboratory medicine although instruments are not operated by laboratory personnel. In this study, we describe the planning and insertion of regulated policies for POCT and quality management, outside of the clinical laboratory. Our results emphasize the importance of the clinical laboratory department involvement to ensure accountable and accurate results in POCT testing.
by Dr Judith Attias, Svetlana Timoshchuk and Dr Marielle Kaplan
Introduction
Point-of-care testing (POCT) refers to tests conducted outside of the central clinical laboratory division. Point-of-care (POC) tests are performed mainly by clinical staff (nurses, physicians, respiratory therapists, etc) and not by clinical lab medicine specialists who understand and work in compliance with quality control (QC) and quality assurance (QA) practices [1]. The major advantage of POCT is the improvement in turnaround time (TAT) of the results by removing transport and clinical lab processing times [2]. As a result, the global POCT market is growing steadily in recent years and it is expected to grow from 23.16 billion USD in 2016 to 36.96 billion USD in 2021 [3]. POCT can be performed in primary, secondary or tertiary healthcare institutions. The list of tests that are permitted to be performed outside the clinical lab differs from one country to another, as do the requirements for quality management, ISO 22870 insertion included [4]. In Europe, current POCT exists for complete blood count including five-part differential, pregnancy testing, blood glucose concentration, cardiac biomarkers, coagulation testing, platelet function, group A streptococcus, HIV testing, malaria screening, etc.
In Israel, a list of the tests allowed to be performed as POC tests (published by the Ministry of Health), as well as the QA requirement exists but, in fact, until recently no policy was applied for POCT insertion and specialist involvement (clinical lab staff and biomedical engineering).
The aim of our work was to list all POCT conducted in a major hospital (1000 beds) located in the north of Israel, to insert a policy for device insertion, to plan and insert a QC and QA programme and to determinate the role of the clinical lab department.
Methods
First a list of all the POCT devices dispersed all over the hospital departments was prepared by the clinical lab department while building up a strong collaboration with the biomedical engineering unit, thus allowing a multidisciplinary approach.
All the blood gas instruments were replaced by GEM family devices to ensure standardization, and connected to the hospital laboratory information system (LIS), as were the glucometers.
Then a policy for POCT insertion was written. A committee composed of representatives of the clinical lab department directors, the biomedical engineering directors and directors from the department where the POCT procedure was to be employed was formed each time. The definition of the committee’s role was to check the relevance of new POCT device insertion from professional and economic aspects.
A policy for QC performance and frequency was adopted. Four quality indices were adopted and reviewed annually:
- Optimization of the use of tests in the cartridge for blood gas.
- Cancellation of test as a result of wrong identification of the patients.
- For glucometers, performance of three QC levels by the department’s staff once a month.
- For blood gas devices, standardization in comparison to the clinical lab was performed by the lab staff monthly. The ratio between the lab results and the POCT device results was calculated. A range for acceptable results was determined (0.9–1.1 for pH, 0.8–1.2 for PCO2 and PO2).
Results
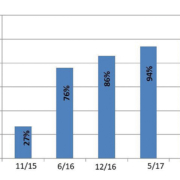
We have now 80 similar glucometers all over the hospital, 10 blood gas instruments from the same family, no general urine instrument (only sticks similar to the sticks used in the clinical lab) and 2 thromboelastograms (TEGs). At the start of the process only 27% of the departments performed glucose QC. After training, the percentage of departments performing QC had grown to 76%. At the end of 2017, we decided that glucose tests would not be done without QC or if the QC results did not meet the expected target of 100% QC performance (Fig. 1). In 2015, there were seven blood gas analysers in five different departments; the instruments were from three different companies and were not connected to the LIS. Now, ten blood gas devices similar to those used in the clinical lab (GEM family instrument which performed QC after every test) are connected to the LIS and dispersed in eight departments. The clinical lab staff audits the use of reagents annually and performs standardization in comparison to the clinical lab once a month. The results show that not all the reagents are used optimally in all departments. The Ambulatory Operating Theatre (Ambulatory OP, which includes outpatient surgery as well as elective caesarean sections) and Intensive Cardiac Care Unit (ICCU) used only 14% and 56% of the reagents, respectively, in 2017 (Fig. 2). The performance level of the instruments is good for the all instruments. Figure three show the standardization results of the Children’s Intensive Care Unit comparatively to the clinical lab results (Fig. 3). No irregularity was obtained. POCT result cancellation is performed only after a clinician’s request to the clinical lab division by mail to the clinical lab staff.
The cancellation percentage as a result of wrong identification in the different departments is low, less than 1% of the totals tests performed (data not shown). The two thromboelastograms are in the open heart surgery theatre and are mainly used by one specific anesthetist. The anesthetist performs internal QC once a week and also participates in an external QC programme. The clinical lab prepares the specimen to be analysed and the anesthetist perform the tests. The clinical lab staff then receive and review the results. The results demonstrate that the results are acceptable but not always performed in the time limit required.
From 2015 to 2017, three new blood gas devices were inserted in the Intensive Care Unit (ICU), ICCU and ambulatory surgery theatre via the POCT device committee. In 2017 the delivery room requested a blood gas device. The number of blood gas tests ordered monthly by this department was reviewed and found to be low. As the clinical lab agreed to give priority to this department for very quick results, the request for a POC blood gas device in the delivery room was rejected by the POCT committee. At the beginning of 2018, a POCT coagulation device was inserted in one department without any consultation with the POCT committee. As in Israel partial thromboplastin time is not a part of the POC allowed tests, the instrument was removed immediately as a result of the committee’s intervention.
Discussion and Conclusion
Our results demonstrate that clinical lab involvement in POCT management led to QC performance and increase QA and insertion procedure supervision. Our results demonstrate that POCT device insertion may be considerate even when it means no optimum reagent use (depending on the need of immediate results). Laboratories all over the world work according to strict QA standard and improve continually QC performance and QC review to ensure results quality. In clinical lab testing, the majority of quality errors occur in the preanalytical phase, which is performed outside the laboratory [5]. In contrast, for POCT the majority of quality errors occurred in the analytic phase [6]. There is no doubt that POCT reduces TAT but we must consider if there is a price to pay and ensure that we do not significantly lower quality. Catherine Zimmerman proposes in her review to improve clinical lab TAT by reducing the transport time of the tubes to the clinical lab and the analytic processes inside the lab, as well as at the post-analytic phase [7]. Another aspect of this issue is whether POCT improves clinical parameters significantly; for example, the length of patient stay in the Emergency Department or mortality. Some results show that rapid results with POCT did not necessarily lead to shorter stays in the Emergency Department [8]. Larsson et al. show that when properly used, POCT improves patient care, workflow and even provides significant financial benefits [9]. They also agree with Pecoraro et al. who demonstrate that further studies may be required for defining the real utility of POCT on clinical decision making [10].
Our results show only a few cancellations owing to wrong patient identification. It is important to take into consideration the possibility of underestimation because of underreporting and unknown errors. In the clinical lab, results are reviewed before release to the clinicians. If an error of any kind is suspected (for example, identification error), the clinical lab staff call the physician, inquire about the quality of the sample and ask for a new sample if there is any doubt. The source of POCT errors are usually operator incompetence, not adhering to test procedures, and use of uncontrolled reagents and devices. Consequences of POCT error may affect patient management decisions and treatment [11]. In the literature, we cannot find data about the percentage of POCT error, especially when the clinical lab is not involved in the processes.
In conclusion, in a world where everything is happening so fast and any data can be obtained so quickly, the real challenge for the clinical lab profession is to overcome POCT antagonism and, on the contrary, to be involved with and supervise all POCT processes.
References
1. Shaw JLV. Practical challenges related to point of care testing. Pract Lab Med 2015; 4: 22–29.
2. Lee-Lewandrowski E, Corboy D, Lewandorwski K, Sinclair J, McDermot S, Benzer TI. Implementation of a point-of-care satellite laboratory in the Emergency Department of an academic medical center. Arch Pathol Lab Med 2003; 127: 456–460.
3. Vashist SK. Point-of-care diagnostics: recent advances and trends. Biosensors 2017; 7(4): 62–65.
4. Boursier G, Vukasovic I, Brguljan PM, Lohmander M, Ghita I, Bernabeu Andreu FA, Barrett E, Brugnoni D, et al. Accreditation process in European countries and EFLM survey. Clin Chem Lab Med 2016; 54(4): 545–551.
5. Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2002; 48: 691–698.
6.O’Kane MJ, McManus P, McGowan N, Lynch PLM. Quality error rates in point of care testing. Clin Chem 2011; 57(9): 1267–1271
7. Zimmermann-Ivol C. POCT aux urgencies: gain de temps ou perte de gain? Pipette Swiss Lab Med 2012; 8–10.
8. Florkowski C, Don-Wauchop A, Gimenez N, Rodriguez-Capot K Wils J, Zemlin A. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM) – does it leverage any advantage in clinical decision making? Clin Lab Sci 2017; 54(7–8): 471–494.
9.Larsson A, Greig-Pylypczuk R, Huisman A. The state of point-of-care testing: a European perspective. Ups J Med Sci 2015; 120(1): 1–10.
10. Pecoraro V, Germagnoli L, Banfi G. Point-of-care testing: where is the evidence? A systematic survey. Clin Chem Lab Med 2014; 52(3): 313–324.
11. Meir FA, Jones BA. Point-of-care testing error: sources and amplifiers, taxonomy, prevention strategies, and detection monitors. Arch Pathol Lab Med 2005; 129: 1262–1267.
The authors
Judith Attias* PhD, Svetlana Timoshchuk and Marielle Kaplan PhD
Rambam Health Care Campus,
POB9602, Haifa 3109601, Israel
*Corresponding author
E-mail: J_attias@rmc.gov.il