Point-of-care glucose meters: useful in a neonatal setting
Point-of-care glucose meters are used in a variety of settings to monitor glucose concentration in whole blood. Comparability between the results from these meters and results issued on plasma samples was examined by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), which in 2006 recommended that all glucose results should be reported as a plasma concentration. The group advised that a conversion factor of 1.11 be used to convert whole blood results to plasma equivalence. As neonatal hematocrit differs from that seen in adults, the IFCC recommendation is not appropriate in neonatal samples. It was decided to review this recommendation.
by Mary Stapleton and Ruth O’Kelly
Introduction
Neonates may be at risk of hypoglycemia in the first few hours and days after birth, the cause of which may be attributed to the stress of extra-uterine life [1]. However, it may also signal an underlying pathology, and prolonged episodes of hypoglycemia have been described as a cause of neurodevelopmental morbidity [2]. Identification of hypoglycemic episodes is, therefore, considered to be vital in the neonatal period, but the population in question often includes extremely premature and small infants. By regularly using point-of-care (POC) devices to measure glucose in this cohort of patients, it is hoped to obtain useful results while avoiding unnecessary blood loss.
In instances where glucose results obtained on POC devices do not fit the clinical picture, a fluoride-preserved sample may be sent for plasma analysis.
Discrepancies between POC whole blood and laboratory plasma results may be a cause of lack of confidence in bedside technology. There are several causes of such discrepancies, and while literature has suggested that hypoglycemia is missed by using POC devices, the role of glycolysis as a pre-analytical factor is starting to be recognized [3]. The second possible cause is that differing sample types are measured and unlikely to be comparable. In 2006, the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) published a recommendation that manufacturers of POC devices were to report glucose concentration as though it were a plasma sample rather than whole blood. A conversion factor of 1.11 was calculated to equate the results from the two sample types (whole blood × 1.11 = plasma) [4].
The aim of this study was to perform glucose measurements in neonatal and adult whole blood and plasma samples by a laboratory method and a POC method without a plasma correction factor. By comparing results, it was hoped to determine the appropriateness of the plasma conversion factor as recommended by the IFCC.
Methods
The HemoCue 201+ POC methodology that was used to analyse whole blood samples consists of an analyser and measuring cuvette containing dried reagents. The cuvette serves as a pipette, reaction chamber and measuring vessel. Analysis of plasma for glucose concentration was performed on an automated chemistry platform (Beckman Coulter AU640) using a hexokinase method in a laboratory accredited to ISO 15189 standards.
Samples for plasma glucose analysis were obtained in tubes containing fluoride as an antiglycolytic agent. When measuring glucose in the POC device, an aliquot of sample was taken from the sample in the blood tube before separation.
Statistical analysis, using Bland–Altman analysis to compare results by two different methods, was performed using Analyse-It software for Microsoft Excel (Analyse-It Software Ltd).
Study 1
Fluoride-stabilized plasma samples from 25 neonates (aged 3 days or less) received into the laboratory for routine glucose estimation were included in the study. An aliquot was taken from each sample before centrifugation and analysis, and glucose determination by POC was performed on a HemoCue 201+ analyser located in the laboratory.
Study 2
Fluoride plasma samples from pregnant women (n = 34) were also analysed for whole blood and plasma glucose in the same manner described in study 1.
Study 3
A portion of patients who were a part of the study had a sample sent for full blood count (FBC) analysis on the same day of the glucose request. Results were subdivided into greater and less than the median result for both hematocrit and mean corpuscular volume (MCV). These were then reviewed against the reported glucose concentrations.
Results
Studies 1 and 2
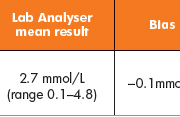
No significant difference was noted between neonatal samples analysed (Table 1, Fig. 1) (bias, 0.05mmol/L). However, a significant difference (P<0.0001) was noted between the two methods when samples had been obtained from adult patients (Table 2, Fig. 2) (bias, 0.6mmol/L).
Study 3
A standard calculation for determining the percentage of water in blood was reviewed (Equation 1). The data obtained from the FBC samples was used to propose plasma conversion factors for both adult and neonatal patients (Table 3). It was assumed that the median hematocrit in a healthy, non-pregnant adult is 0.43 L/L, with a resulting calculated conversion factor (CCF) of 1.11.
Discussion
This study investigated the reported difference between samples analysed for glucose using POC meters in a ward setting and those samples received for glucose analysis in a central laboratory. It may be seen that there is good correlation between POC and laboratory analyser methods in samples obtained from neonates.
This correlation was not seen in the set of adult samples analysed, and an average difference of up to 10% in results was reported from the two methods. By applying a plasma equivalence factor of 1.11 to the whole blood results from adults as recommended by the IFCC in 2006, the difference in results from adult patients could be explained.
The IFCC equivalence factor based on the hematocrit in neonates is 1.15, but this study confirms that the neonatal samples did not require this factor. POC glucose measurements in the HemoCue device include a cell lysis step and thus whole blood (intra-and extra-cellular) glucose is measured. However, neonatal blood is recognized as containing resistant cells and cells may not fully lyse causing the measured glucose to reflect extra-cellular glucose similar to plasma measurements.
In a previous study [5], Vadasdi and Jacobs compared heparinized samples from neonates that were analysed on the HemoCue immediately before centrifugation and assayed by the laboratory method. No significant difference was found between the mean values of the two methods over a hematocrit range of 0.185–0.72. Our study agrees with these findings.
Vadasdi and Jacobs suggested that the effect of hematocrit was decreased significantly by the hemolysis step in the cuvette. It is recognized that HemoCue POC meters are not affected by hematocrit [4, 5], which is why this meter is frequently used in a neonatal setting. Vadasdi and Jacobs also suggested that because the MCV (which describes the size of the red cells) is greater than seen in adults, there is less of a dilutional effect due to membrane proteins after lysis. Our study showed that the mean MCV in neonates was greater than seen in our adult (pregnant) subjects.
Conclusion
Laboratory measurements for glucose are usually performed on plasma samples while POC measurements are performed on whole blood. A difference in results may be expected as whole blood glucose is known to be approximately 11% lower than plasma glucose due to lower volume of water in the erythrocytes.
The difference between plasma and whole blood glucose in adults was similar to the recommended IFCC “plasma equivalent factor” of 1.11. The lack of difference between plasma and whole blood glucose in neonatal samples may be explained by the increased MCV or the presence of resistant red cells that may not undergo lysis in the POC device.
Many modern POC devices for measuring glucose now include the IFCC plasma conversion factor and such results should be carefully interpreted.
References
1. World Health Organization. Hypoglycaemia of the newborn. Review of the literature. WHO/CHD/97.1, 1997.
2. Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988; 297(6659): 1304–1308.
3. Stapleton M, Daly N, O’Kelly R, Turner MJ. Time and temperature affect glycolysis in blood samples regardless of fluoride- based preservatives: a potential underestimation of diabetes. Ann Clin Biochem 2017; 54: 671–676.
4. D’Orazio P, Burnett RW, Fogh-Anderson N, Jacobs E, Kuwa K, Külpmann WR, Larsson L, Lewenstam A, Maas AH, et al. Approved IFCC recommendation on reporting results for blood glucose: International Federation of Clinical Chemistry and Laboratory Medicine Scientific Division, Working Group on Selective Electrodes and Point of Care Testing (IFCC-SD-WG-SEPOCT). Clin Chem Lab Med 2006; 44: 1486–1490.
5. Vadasdi E, Jacobs E. HemoCue β-glucose photometer evaluated for use in a neonatal intensive care unit. Clin Chem 1993; 39(11): 2329–2332.
The authors
Mary Stapleton* FRCPath; Ruth O’Kelly FRCPath
Biochemistry Department, Coombe Women & Infants University Hospital, Dublin, Ireland
*Corresponding author
E-mail: mary.stapleton@nhs.net