Primary hyperoxaluria and measurement of relevant metabolites
The primary hyperoxalurias are inherited disorders of urine oxalate overproduction that have significant morbidity and mortality. This article briefly reviews the three known disorders, their presentation, biochemical diagnosis and treatment strategies highlighting preanalytical and analytical issues raised with mass spectrometric methodologies.
by Felicity Stokes and Dr Gill Rumsby
Introduction
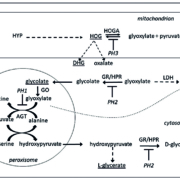
The primary hyperoxalurias (PHs) are inherited disorders of endogenous oxalate overproduction characterized by excessive urinary oxalate excretion causing recurrent calcium oxalate kidney stones and/or nephrocalcinosis, ultimately leading to renal failure and systemic deposition of oxalate. There are at least three types of PH caused by specific enzyme deficiencies (Fig. 1). PH1 is a deficiency of the hepatic peroxisomal enzyme, alanine: glyoxylate aminotransferase (AGT); PH2 is caused by defects of glyoxylate/hydroxypyruvate reductase (GR/HPR), a cytosolic enzyme with a wider tissue distribution but predominantly in the liver; the third disorder, PH3, is the result of defects in the mitochondrial enzyme hydroxyoxoglutarate aldolase (HOGA) that is found in the liver and kidney. AGT and GR/HPR play a major role in the metabolism (detoxification) of glyoxylate, a 2-carbon keto acid produced from glycolate (Fig. 2), and deficiency of either of the enzymes leads to glyoxylate accumulation and its metabolism to oxalate by the enzyme lactate dehydrogenase (LDH). The mechanism of oxalate production in PH3 is as yet unknown as glyoxylate is actually a product of the normal enzyme reaction but urinary oxalate can be as high as in the other two forms of PH [1].
The majority of cases of PH present in childhood and diagnosis relies on the demonstration of hyperoxaluria, but diagnosis can be frustrated by the high biological variability of urinary oxalate, the need to collect 24-h urine samples and proper acidification of samples. Random samples, where results are expressed relative to urinary creatinine, can be used but are more prone to dietary influences on oxalate excretion and it is recommended to repeat the analysis to confirm, potentially leading to delays in diagnosis. Secondary hyperoxaluria, caused by excessive oxalate uptake, is a more common finding and therefore dietary excess as well as disorders affecting the gastrointestinal tract and pancreatic function need to be excluded. In cases where end-stage renal disease has already occurred, urine oxalate is unreliable and the patient may be anuric; therefore, measurement of plasma oxalate is required. It should be added that this is the only time that plasma oxalate is worth measuring.
PH1, PH2 and PH3 can be further differentiated by analysis of urine metabolites characteristic of the three disorders. These PH metabolites (PHM) are glycolate, glycerate, 4-hydroxy-2-oxoglutarate (HOG) and 2,4-dihydroxyglutarate (DHG) (Fig. 2). Glycolate is elevated in more than two-thirds of cases of PH1 at concentrations similar to those of oxalate but its absence cannot exclude the disease. It is not clear why some PH1 individuals maintain levels of glycolate within the reference range but it may reflect individual differences in activity of the glycolate oxidase enzyme. L-glycerate, produced by the action of LDH on hydroxypyruvate, is raised in the vast majority of cases of PH2 and its finding in individuals with hyperoxaluria is pathognomonic for the disorder. Its occasional reported absence may reflect methodological differences with poor extraction of this analyte in organic acid screens. HOG and its reduced metabolite, DHG, are both found in PH3 although the latter is a more reliable indicator [2, 3]. The finding of particular patterns of elevated metabolites allows focusing of genetic testing and confirmation of the diagnosis by sequencing of the relevant gene. This strategy also permits further testing of possibly asymptomatic family members (potential living-related organ donors) and prenatal testing.
Treatment for these disorders initially involves conservative therapy including high fluid intake, and citrate and orthophosphate to act as calcium binders to reduce calcium oxalate supersaturation. Some cases of PH1 will respond to pyridoxine supplementation (a cofactor for AGT), which reduces urine oxalate excretion to normal levels. For those patients in renal failure, transplantation is required to prevent the long-term problems associated with dialysis and systemic oxalosis. For PH1 a liver or combined liver-kidney transplant are the preferred treatments, whereas for PH2 although renal transplants alone have been used, these do not provide a cure and liver-kidney transplant may need to be considered. As yet only two cases of PH3 in renal failure have been published and the long-term outcome and optimum treatment of this disorder is therefore unknown.
Novel treatments, currently in clinical trials, are substrate reduction strategies by RNA interference and include blocking glycolate oxidase to prevent glyoxylate formation and inhibition of LDH to prevent oxalate synthesis. These and other potential strategies have been recently reviewed [4].
Biochemical testing for PHM
Gas chromatography-mass spectrometry
Analysis of PHM by gas chromatography-mass spectrometry (GC-MS) requires derivatization of the metabolites to protect thermally labile groups from the high temperatures required for volatilization and separation. Methoxyamine and N-trimethylsilylimidazole (MO-TMS reagent) can be used for this purpose; the carbonyl group on 4-hydroxy-2-oxoglutarate is protected by the methoxymation reaction followed by silylation of hydroxyl groups to create trimethylsilyl ethers. Several extractions are required to isolate the derivatives before separation on a non-polar HP-5ms capillary column with detection of products by selective ion monitoring. With the exception of d2-labelled glycolate, none of the other PH metabolites are available as isotopically labelled internal standards, hence dimethylglutarate was used as an additional internal standard. There are also no certified calibrators for these metabolites and thus it is essential to establish local reference ranges with appropriate cut-offs for a provisional diagnosis of PH. Recoveries of metabolites ranged from 91 to 103 % with analytical imprecision ranging from 3 to 13.6 %.
DHG elutes as two distinct peaks on GC-MS: one is the meso isomer (2S,4R-DHG), the other a mixture of stereoisomers (2S, 4S- and 2R, 4R-DHG). The stereoisomer peak was consistently greater in all patients with PH3 but both peaks are summed to give a total DHG concentration. The method also does not discriminate between L- and D-glycerate but L-glycericaciduria (i.e. PH2) and D-glycericaciduria have very different presentations and this is unlikely to be an issue. HOG was found to be unstable if urine samples were not acidified at time of collection, leading to potential false negatives.
Liquid chromatography-tandem mass spectrometry The simultaneous measurement of glycolate, glycerate and DHG by liquid chromatography-tandem mass spectrometry (LC-MS/MS) can be carried out with a significantly shortened and simplified sample preparation as compared to GC-MS [3]. Urine samples are diluted 1:1 with internal standard followed by microfiltration. A 1 µL injection onto a Phenomenex Luna Omega PS C18 column retains these relatively polar analytes, with all three analytes and the internal standard (d2-glycolate) co-eluting at 2.9 minutes. The problems with the preanalytical stability of HOG mentioned above convinced us not to include measurement of this metabolite by LC-MS/MS.
Validation of this assay showed a mean recovery of 103, 104 and 93 % and a mean total imprecision of 6.4, 10 and 11 % coefficient of variation (CV) for glycolate, glycerate and DHG respectively. The assay has a wide measuring range for each analyte with lower limit of quantitation (CV<20 %) of 76, 20 and 1 µmol/L for glycolate, glycerate and DHG and linearity up to 3000 µmol/L for glycolate, 2000 µmol/L for glycerate and 300 µmol/L for DHG.
LC-MS/MS results compared well with GC-MS for glycolate and DHG (mean bias 3.3 and 5.7 %). The mean glycerate bias was greater (−8.7 %) but in the absence of certified reference materials, it is impossible to know the true accuracy of these methods. Diagnostic cut-offs have been developed and validated through the analysis of samples from controls and patients with genetically confirmed PH1, 2 and 3 using ROC analysis.
A specificity and sensitivity of 100 % were achieved using a cut-off of 100 µmol glycerate/mmol creatinine and 5 µmol DHG/mmol creatinine for PH2 and PH3, respectively. DHG was a much more reliable marker of PH3 than HOG, the latter having only 76 % sensitivity. Mild elevations of urinary glycolate can derive from the diet and an overlap was seen in patients with PH1 and those without (Fig. 3). A cut-off of 193 µmol glycolate/mmol creatinine gave 100 % specificity for PH1, albeit with a reduced sensitivity of 73 %.
Limitations of methods
The major limitation of the GC-MS method was the need for derivatization. For LC-MS/MS, the quick, but fairly crude, sample preparation is influenced by the heterogeneous matrix of urine which can, in some samples, result in interference in the retention of analytes by an unknown mechanism. Experiments have shown that this may be related to differences in urea and ammonia concentration in urine samples. A modification to our original published method, using a solid phase extraction technique, is currently being evaluated in order to try to standardize the matrix injected into the column.
Conclusion
GC-MS and LC-MS/MS have both proven useful for a provisional diagnosis of PH, the latter offering a rapid method. Both methodologies are limited by the lack of specific internal standards and certified calibrators but these limitations can be overcome by co-elution of the metabolites and the use of locally derived reference ranges.
Figure 1. Metabolic pathways affected by primary hyperoxaluria (PH)1, PH2 and PH3.
The metabolites measured by GC-MS and LC-MS/MS are underlined.
AGT, alanine:glyoxylate aminotransferase; DHG, 2,4-dihydroxyglutarate; GR/HPR, glyoxylate/hydroxypyruvate reductase; GO, glycolate oxidase; HOG, 4-hydroxy-2-oxoglutarate; HOGA, hydroxyoxoglutarate aldolase; HYP, hydroxyproline; LDH, lactate dehydrogenase.
Figure 2. Structure of the biochemical metabolites produced in excess in PH.
Figure 3. Clinical value of PH metabolites analysis by LC-MS/MS for the diagnosis of PH.
*P>0.05; **P>0.01;***P>0.001 (Redrawn from Woodward G, et al. Rapid liquid chromatography tandem mass-spectrometry screening method for urinary metabolites of primary hyperoxaluria. Ann Clin Biochem 2019; 56(2): 232–239 [3].)
References
1. Clifford-Mobley O, Tims C, Rumsby G. The comparability of oxalate excretion and oxalate:creatinine ratio in the investigation of primary hyperoxaluria:a review of data from a referral centre. Ann Clin Biochem 2015; 52: 113–121.
2. Clifford-Mobley O, Hewitt L, Rumsby G. Simultaneous analysis of urinary metabolites for preliminary identification of primary hyperoxaluria. Ann Clin Biochem 2016; 53(4): 485–494.
3. Woodward G, Pryke R, Hoppe B, Rumsby G. Rapid liquid chromatography tandem mass-spectrometry screening method for urinary metabolites of primary hyperoxaluria. Ann Clin Biochem 2019; 56(2): 232–239.
4. Rumsby G, Hulton S-A. From pathogenesis to novel therapies in primary hyperoxaluria. Expert Opin Orphan Drugs 2019; 7(2): 57–66.
The authors
Felicity Stokes1 BSc, MSc; Gill Rumsby*2 PhD, FRCPath
1Manual Blood Sciences, HSL Analytics LLP, London, UK
2Retired; formerly Consultant Clinical Scientist, Clinical Biochemistry, UCL Hospitals, London, UK
*Corresponding author
E-mail: gillrumsby@btinternet.com