Siemens starts worldwide shipping of total antibody test for COVID-19
Siemens Healthineers announced late May that it is now shipping worldwide its laboratory-based total antibody test to detect the presence of SARS-CoV-2 IgM and IgG antibodies in blood. The test received the CE mark and data has demonstrated 100 percent sensitivity and 99.8 percent specificity. The total antibody test allows for identification of patients who have developed an adaptive immune response, which indicates recent infection or prior exposure.
The US FDA has issued an Emergency Use Authorization (EUA) for its laboratory-based total antibody test.
Siemens says it is prepared to ramp up production as the pandemic evolves with capacity exceeding 50 million tests per month across its platforms starting in June.
The antibody test is now available on the largest installed base in the U.S. and one of the largest in the world with 20,000 Siemens Healthineers systems installed worldwide. This includes the Atellica Solution immunoassay analyser, which can run up to 440 tests per hour and enables a result in just 10 minutes. By detecting both IgM and IgG antibodies, the test provides a clearer clinical picture over a longer period of time as the disease progresses.
The antibody test also is available on the company’s installed base of ADVIA Centaur XP and XPT analysers, which can test up to 240 samples per hour, with a result in 18 minutes.
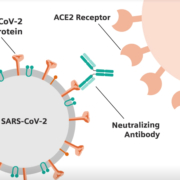
Importantly, the test detects antibodies to a key spike protein on the surface of the SARS-CoV-2 virus, which binds the virus to cells with a distinct human receptor found in lungs, heart, multiple organs and blood vessels. Studies indicate that certain (neutralizing) antibodies to the spike protein can disarm SARS-CoV-2, presumably by interfering with the ability of the virus to bind, penetrate and infect human cells. Multiple potential vaccines in development for SARS-CoV-2 include the spike protein within their focus.