Steroid testing with the Triple Quad mass spectrometer: profiling with the Gold Standard
In the human body, steroid hormones are involved in a variety of regulatory processes, which makes them also important diagnostic markers for a range of diseases. However, due to their high chemical similarity, they can represent a challenge for many assays – immunoassays in particular suffer from cross-reactivities. In comparison, LC-MS/MS-based assays provide high specificity in combination with the ability to determine several steroids in one run.
by Dr Marc Egelhofer
Steroids have a common distinct chemical structure – they consist of a cholesterol backbone with 3 hexane rings and a pentane ring. The hormones are synthesized in the adrenal cortex (corticosteroids) as well as in the reproductive organs (androgens, estrogens). Several doping agents are also artificial derivatives of the male sexual hormone testosterone, called anabolics, and are used abusively to increase muscle and bone synthesis.
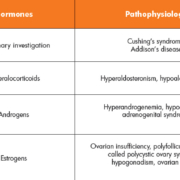
With a distinguished role in regulatory processes of the human body, dysfunctional steroid release can be responsible for many diseases with sometimes extremely unspecific symptoms (see Table 1). One example is aldosteronism, where the adrenal glands produce excessive amounts of the steroid hormone aldosterone. This leads to lowered levels of potassium in the blood (hypokalemia) and an increased excretion of hydrogen ions (alkalosis). Patients suffer from muscle spasms, fatigue, headaches, high blood pressure, and muscle weakness. However, these symptoms can be attributed to many diseases, and only the clinical evaluation of aldosterone plasma levels can ensure a correct diagnosis.
Challenging targets
The chemical similarity of the steroid structure can be a challenge, in particular in a clinical setting where requirements in specificity and selectivity need to be met. This problem becomes evident when looking at epidemiological studies of major diseases, where many different assay methods with a varying performance are used, resulting in an inability to compare data [1]. The discrepancies in assay performance also limit investigations where comparisons of absolute steroid concentration values are used, rather than relative levels. For example, absolute steroid hormone concentrations are needed when analysing effects of hormonal threshold concentrations to obtain a certain disease outcome – or not.
Steroid profiling
A lot of the published literature and of our knowledge about the physiology of steroid hormones is based on radioimmunoassays (RIA). One of the reasons for discrepancies in values, however, is that immunoassays suffer from various interferences due to antibody cross-reactions with other steroid hormones. In contrast, mass spectrometry has been recognized as the best available method for the accurate analysis of steroids in biological samples [2]. It overcomes limitations of immunoassays, while also simplifying the sample preparation in comparison to GC-MS/MS analysis that requires lengthy derivatization processes to obtain the analytes in the gaseous phase for separation.
We have developed a CE-IVD assay for mass spectrometry (MassChrom Steroids) for the determination of 15 steroid hormones. The subsequent analysis takes place in multi reaction monitoring mode (MRM). In this mode, the first and second mass spectrometers are set to a fixed certain mass. MS1 selects only the molecular ion, and ions with a different mass are disregarded. The molecular ion then fragments in the collision cell and MS2 detects the characteristic fragment. The MRM mode makes it possible to determine several steroids in a single run, thereby reducing the time for analysis and increasing the effectiveness of the method. The 15 hormones that can be analysed with this method are divided into two panels for a clear separation of each of the analytes (see Figure 1).
The chromatographic setup, including the analytical column, is identical for all analytes, thereby eliminating the need to change columns or mobile phases between separate runs. Depending on requirements and throughput, sample preparation can be performed in 96 SPE well plates or SPE columns. The assay has been tested on a range of systems, such as the AB Sciex Triple Quad 4500 or the Waters Xevo TQS instruments.
Salivary sampling
Plasma sampling can represent a problem, in particular for parameters that need to be collected several times a day or under stress-free conditions. Saliva consists of 99.5% water, electrolytes, mucus, white blood cells, epithelial cells, glycoproteins and enzymes, though saliva is also a carrier of steroid hormones. The speed at which they are transferred from blood into saliva is controlled by passage through the lipophilic layers of the capillaries and glandular epithelial cells. Consequently, the more lipophilic the molecules the faster is the transfer through these barriers. Salivary concentrations are therefore dependent on the lipophilic properties of the molecule — lipid-soluble steroids such as cortisol have higher concentrations, whereas more hydrophilic substances such as dehydroepiandrosterone-sulfate (DHEA-S) have much lower concentrations relative to the free plasma levels [3].
One of the common medical indications of cortisol testing in saliva is the screening for Cushing’s syndrome, a pathological increase of cortisol [4]. This hypercortisolism can be due to an endogenous overproduction or based on the intake of exogenous glucocorticoids. Symptoms may include obesity, hypertension, hyperglycemia, muscle weakness and osteoporosis. However, these symptoms are also not specific – the majority of individuals with some or all of the symptoms will not suffer from Cushing’s syndrome, therefore, the analysis of cortisol plays a significant role in the identification of the disease.
Cortisol levels do vary significantly over the course of the day (see Figure 2), making it a requirement to measure several times a day. Salivary sampling represents a simple, non-invasive and, for the patient, stress-free sampling method [5]. After a short introduction, patients can collect their sample by themselves at home, which results in a simple process to obtain samples at different stages of the circadian cycle.
The non-invasive nature of the collection procedure also enables samples to be obtained from patients afraid of venipuncture without provoking an unwanted adrenal stress response, especially in children and phobic patients. A disturbing influence of stress-induced adrenal activity is less likely, making salivary sampling more reliable compared with serum, in particular in stress research and pediatric applications [3].
We have developed a CE-IVD method for the determination of cortisol and cortisone in saliva with a sample prep procedure that is performed by filtration and in just a few steps (see Table 2).
The use of stable isotopically labelled internal standards for both analytes ensures reproducible and reliable quantification of the parameters. The performance data are 96-105% for the recovery of spiked samples, an intraassay variation of CV = 2-5%, and interassay variation of CV = 2-7 %, and the lower limit of quantification is 0.27 µg/l (see Figure 3).
Conclusions
Immunoassays are widely used for the measurement of steroids, though it is accepted that these methods suffer from various interferences due to antibody cross-reactions with other steroid hormones. In contrast, LC-MS/MS has been recognized as the best available method for the accurate analysis of steroids in biological samples. LC-MS/MS overcomes many limitations of immunoassays, enhances diagnostic utility of the testing, and expands diagnostic capabilities in endocrinology. In addition to the superior quality of the measurements, LC-MS/MS can help in the standardization and harmonization of steroid testing among clinical laboratories. Commercial suppliers offer complete solutions from sample to result that allow the determination of steroids with LC-MS/MS as the gold standard and without the need to go through the development of an in-house method.
References
1. Stanczyk F. et al. Standardization of Steroid Hormone Assays: Why, How and When? Cancer Epidemiol. Biomarkers Prev. 2017; 16(9): 1713-1719.
2. Rosner W. et al. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2017; 92(2): 405-13.
3. Gröschl M. Current Status of Salivary Hormone Analysis Clin. Chem. 2008; 1759 54(11): 1759-69.
4. Nieman L.K. et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008; 93(5):1526-40.
5. De Palo EF et al. Human saliva cortisone and cortisol simultaneous analysis using reverse phase HPLC technique. Clin Chim Acta. 2009; 405(1-2): 60-5.
The author
Marc Egelhofer PhD*
Chromsystems Instruments & Chemicals GmbH, Am Haag 12, 82166 Gräfelfing, Germany
*Corresponding author,
egelhofer@chromsystems.de