Urine ethyl glucuronide and ethyl sulphate measurement using liquid chromatography-tandem mass spectrometry
Background
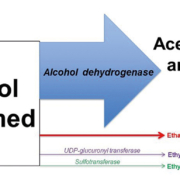
Ethyl glucuronide (EtG) and ethyl sulphate (EtS) are minor ethanol metabolites that can be used to detect recent alcohol consumption [1, 2]. Following the ingestion of alcohol, over 95% is metabolized by alcohol dehydrogenase to acetaldehyde. Up to 5% of ethanol is excreted unchanged in breath, sweat and urine. A small amount of ethanol (<0.1%) is metabolized in the liver by conjugation of glucuronic acid or sulphate to form EtG and EtS (Fig. 1). Following alcohol consumption, ethanol itself can only be detected in breath or urine for up to 6 or 12 hours, respectively (depending on the amount of alcohol consumed) [3]. In comparison, it has been demonstrated that EtG and EtS can be detected in urine for at least 24 hours and over 48 hours with heavy alcohol consumption [4].
The ability of these markers to detect alcohol intake over a longer time period means that they can be useful to identify alcohol relapses in alcohol-dependent individuals in treatment programmes [5]. In the UK, alcohol treatment programmes rely on breath ethanol and self-reporting to detect recent alcohol intake. However, this will only detect a proportion of individuals who are continuing to drink alcohol; this has been a low as 7% in one study comparing breathalyser/self-reported alcohol intake to urine EtG measurement [6]. Therefore, EtG and EtS can be helpful to detect those in alcohol treatment who are continuing to drink alcohol but deny it and have a negative breath ethanol test [7]. This allows additional interventions in individuals who are continuing to drink, which may ultimately improve outcomes. During 2016–17, 80 454 individuals entered alcohol treatment in England; of those 61% were free of alcohol dependence following the standard 12-week programme [8]. Therefore, improved detection of continuing alcohol consumption could lead to initiation of earlier intervention and altered strategies to increase the numbers successfully completing treatment.
Measurement of ethyl glucuronide and ethyl sulphate
Liquid chromatography (LC) to separate analytes with detection using mass spectrometry (MS) is now routinely used in clinical laboratories for an increasing number of tests. It is routine practice in urine toxicology testing for results to be confirmed by either LC or gas chromatography with detection using MS and it has been recommended by the United States Substance Abuse and Mental Health Services Administration (SAMHSA) that MS confirmation should be used for the measurement of EtG and EtS [9].
In tandem MS, two mass spectrometers are arranged sequentially with a ‘collision cell’ placed between the two instruments (Fig. 2). Using selective reaction monitoring, the first mass spectrometer (MS1) selects the ion with the mass/charge (m/z) ratio of interest. The selected ion (parent ion) is fragmented into small ions that enter the second mass spectrometer (MS2) where an ion with a specific m/z ratio is selected (daughter). Detection of analytes using an m/z ratio is very specific and sensitive allowing detection of very small amounts of EtG and EtS.
A number of liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods for EtG and EtS have been published and a reference method has been proposed for EtG using solid phase extraction followed by LC-MS/MS [10]. Deuterated standards (EtG-d5 and EtS-d5) are readily available to purchase for use as internal standards ensuring reproducibility and reliability; an internal standard must mimic the analyte of interest but have a different mass to allow the MS detector to differentiate between the analyte of interest and the internal standard.
Sample preparation in published methods ranges from solid phase extraction to protein precipitation to dilution of urine in mobile phase. Solid phase extraction or protein precipitation of urine samples prior to LC-MS/MS can reduce the presence of potentially interfering substances which may cause ion suppression. It may also help to increase the lifespan of the column. For chromatographic separation of EtG and EtS, the mobile phases are usually formic acid in HPLC grade water and acetonitrile. Published methods have used both isocratic and gradients of mobile phase A and B to achieve separation of EtG and EtS; this is dependent on the sample preparation, the exact composition of the mobile phases and the column chosen. A rapid sample preparation of diluting urine samples in mobile phase A and then adding internal standard has been shown to be effective with no ion suppression or enhancement at or near the retention times for EtG and EtS [11]. Our experience has been to use an increasing gradient of mobile phase B (acetonitrile) from 1% to 10% over the first 2 minutes and then 10% to 100% from 2 minutes to 2.5 minutes. The increase from 1% to 10% acetonitrile elutes EtS/EtS-d5 at 1.27 minutes and the increase from 10% to 100% elutes EtG/EtG-d5 at 2.03 minutes. Figure 3 shows an example chromatogram for a urine sample collected from an individual attending the community based alcohol treatment programme; the high EtG and EtS results demonstrate that this person was continuing to drink alcohol.
Using MS to measure EtG and EtS requires the availability of LC-MS/MS equipment within the laboratory, the technical expertise required to set up an LC-MS/MS method and a dedicated member of staff to perform the analysis. In laboratories already using LC-MS/MS for other assays, there should be no difficulty in setting up a method to measure urine EtG and EtS.
An enzyme immunoassay method is also available to measure EtG and may be adapted for use on many automated laboratory analysers. This method has been shown to compare well to an LC-MS method [12]. For routine use, an immunoassay for EtG on an automated analyser has a number of advantages including rapid turnaround times, availability of EtG analysis out of routine working hours and the same staff members performing the analyses of multiple tests at the same time. However, there is no requirement for urine EtG and EtS analysis to be performed 24/7 as they would not be required in an acute setting. Generally, clients in a community treatment programme attend weekly, so once or twice weekly analysis using LC-MS/MS should be adequate for feedback of results to clients at their next visit. Not requiring a dedicated member of staff (as would be required for LC-MS/MS) is advantageous but according to SAMHSA guidelines, immunoassay results will require confirmation using a MS method. In addition, there is currently no immunoassay method available to measure EtS. This is important as there are a number of scenarios that can cause a false positive EtG result with a negative EtS result. For example, ‘positive’ EtG results (but not EtS results) have been demonstrated after the consumption of non-alcoholic beers (alcohol content 0.5%) [13]. EtG could also be formed in subjects with glycosuria and E.coli infection. If ethanol was formed due to the fermentation of sugars in the urine, this could be converted to EtG by bacteria present in the urine [14]. EtS would not be produced so again EtS can verify whether the EtG result is a true positive. Both EtG and EtS have been detected in individuals who used ethanol-based mouthwash or hand gel; however, the mouthwash was gargled 4 times/day which is much higher than the recommended frequency of use [15]. Owing to these factors, it is advisable to measure both EtG and EtS, which is currently only possible if using LC-MS/MS.
Cut-off values for EtG and EtS
There has been a lot of debate in the literature about suitable cut-off values to use for EtG and EtS. Some authors have suggested using the lower limit of detection (LLOD) or lower limit of quantitation (LLOQ) for the method so that any detectable EtG and EtS is a ‘positive’ result. However, the LLOD and LLOQ in LC-MS/MS methods will be variable between laboratories depending on a number of factors including sample preparation, column choice, chromatography and the tandem MS optimization. For EtG and EtS, the published LLOQs range from 0.05–0.20 mg/L and 0.04–0.10 mg/L respectively. New Clinical & Laboratory Standards Institute (CLSI) guidelines were published in 2016 and these should help to improve standardization between LC-MS/MS methods [16]. Alternatively, cut-off values could be defined by measuring EtG and EtS in a non-drinking population and incorporating measurement uncertainty (0.26 mg/L and 0.22 mg/L for EtG and EtS respectively) [11]. For EtG, a cut-off of 0.50 mg/L has been proposed to reduce the risk of false positive results. The disadvantage of a higher EtG cut-off is a reduction in sensitivity. Jatlow et al. demonstrated that using a 0.50 mg/L cut-off would only detect the intake of a low dose of alcohol 12 hours earlier (estimated blood alcohol 20 mg/dL) in 50% of participants. However, all participants had results above 0.10 mg/L and 0.20 mg/L after the same low alcohol dose 12 hours earlier [4]. SAMHSA have suggested separating EtG results into ‘high’ positive (>1.00 mg/L), ‘low’ positive (0.50–1.00 mg/L) and ‘very low’ positive (0.10–0.50 mg/L). They suggest that a ‘very low’ positive result may indicate previous heavy drinking (1–3 days ago), previous light drinking (12–36 hours ago) or ‘extraneous’ exposure [9].
Another consideration for urine EtG and EtS analysis is the dilution of urine samples; in urine toxicology testing, it is standard practice to measure creatinine to check the validity of a urine sample. There is limited data on the utility of EtG and EtS creatinine ratios. However, it is good practice to measure creatinine and question the validity of the EtG and EtS results if the creatinine is ≤2.0 mmol/L [17].
Conclusion
Urine EtG and EtS are valuable additional tools to detect recent alcohol intake in individuals undergoing treatment for alcohol dependence to ensure continued abstinence. Owing to the risk of false positive EtG results from unintentional exposure (e.g. non-alcoholic beer, urine infection with glycosuria, ethanol-based hand gel/mouthwash), the measurement of EtS in addition to EtG is recommended. An immunoassay is available for EtG but only MS allows the detection of both EtG and EtS to confidently confirm recent alcohol intake. There are a number of published methods for LC-MS/MS for EtG and EtS which are applicable for routine use in a clinical laboratory.
References
1. Dahl H, Stephanson N, Beck O, Helander A. Comparison of urinary excretion characteristics of ethanol and ethyl glucuronide. J Anal Toxicol 2002; 26: 201–204.
2. Helander A, Beck O. Ethyl Sulphate – a metabolite of ethanol in humans and a potential biomarker of acute alcohol intake. J Anal Toxicol 2005; 29: 270–274.
3. Helander A, Beck O, Jones W. Laboratory testing for recent alcohol consumption: comparison of ethanol, methanol and 5-hydroxytryptophol. Clin Chem 1996; 42: 618–624.
4. Jatlow P, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, Nogueira C, Shi J, Dziura JD, et al. Ethylglucuronide and ethyl sulfate assays in clinical trials, interpretation and limitations: results of a dose ranging alcohol challenge study and two clinical trials. Alcohol Clin Exp Res. 2014; 38: 2056–2065.
5. Dahl H, Voltaire Carlsson A, Hillgren K, Helander A. Urinary ethyl glucuronide and ethyl sulphate for detection of recent drinking in an outpatient treatment program for alcohol and drug dependence. Alcohol Alcohol 2011; 46: 278–282.
6. Wetterling T, Dibbelt L, Wetterling G, Göder R, Wurst F, Margraf M, Junghanns K. Ethyl glucuronide (EtG): better than breathalyser or self-reports to detect covert short-term relapses into drinking. Alcohol Alcohol 2014; 49: 51–54.
7. Armer J, Gunawardana L, Allcock R. The performance of alcohol markers including ethyl glucuronide and ethyl sulphate to detect alcohol use in clients in a community alcohol treatment programme. Alcohol Alcohol 2017; 52: 29–34.
8. Knight J, Brand P, Willey P, van der Merwe J. Adult substance misuse statistics from the National Drug Treatment Monitoring System (NDTMS): 01 April 2016 – 31 March 2017. Public Health England 2017
(https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/658056/Adult-statistics-from-the-national-drug-treatment-monitoring-system-2016-2017.pdf).
9. The role of biomarkers in the treatment of alcohol use disorders. Substance Abuse and Mental Health Services Administration (SAMHSA) Advisory 2012; 11(2) (https://store.samhsa.gov/shin/content/SMA12-4686/SMA12-4686.pdf).
10. Helander A, Kenan N, Beck O. Comparison of analytical approaches for liquid chromatography/mass spectrometric determination of the alcohol biomarker ethyl glucuronide in urine. Rapid Commun Mass Spectrom 2010: 24: 1737–1743.
11. Armer J, Allcock R. Urine ethyl glucuronide and ethyl sulphate using liquid chromatography-tandem mass spectrometry in a routine clinical laboratory. Ann Clin Biochem 2017; 54: 60–68.
12. Bottcher M, Beck O, Helander A. Evaluation of a new immunoassay for urine ethyl glucuronide testing. Alcohol Alcohol 2008; 43: 46–48.
13. Thierauf A, Gnann H, Wohlfarth A, Auwärter V, Perdekamp MG, Buttler KJ, Wurst FM, Weinmann W. Urine tested positive for ethyl glucuronide and ethyl sulphate after the consumption of “non-alcoholic” beer. Forensic Sci Int 2010; 202: 82–85.
14. Helander A, Ollson I, Dahl H. Postcollection synthesis of ethyl glucuronide by bacteria in urine may cause false identification of alcohol consumption. Clin Chem 2007; 53: 1855–1857.
15. Reisfield G, Goldberger B, Pesce A, Crews BO, Wilson GR, Teitelbaum SA, Bertholf RL. Ethyl glucuronide, ethyl sulfate, and ethanol in urine after intensive exposure to high ethanol content mouthwash. J Anal Toxicol 2011; 35: 264–268.
16. Lynch K. CLSI C62-A: a new standard for clinical mass spectrometry. Clin Chem 2016; 62(1): 24–29.
17. European guidelines for workplace drug testing in urine. European Workplace Drug Testing Society 2015 (http://www.ewdts.org/data/uploads/documents/ewdts-urine-guideline-2015-11-01-v2.0.pdf).
The authors
Jane Armer*1 BA MSc FRCPath and Rebecca Allcock2 BSc MSc FRCPath
1Department of Blood Sciences, East Lancashire Hospitals NHS Trust, Blackburn, UK
2Department of Clinical Biochemistry, Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK
*Corresponding author
E-mail: jane.oakey@elht.nhs.uk