Variation in measuring and reporting serum indices and the effect on clinical practice
The presence of hemoglobin (hemolysis), bilirubin (icterus) and lipids (lipemia) in serum can affect the measurement and reporting of many clinical chemistry laboratory test results. This is not only in the validity of the test results themselves, but also whether a result is, or should be, reported. Quality assurance of serum indices is not as rigorous as for other clinical chemistry assays. This article highlights the variation in practice which is ultimately affecting the patient and their care pathway.
by Dr Rachel Marrington and Finlay MacKenzie
Introduction
There are a number of interferences that affect the analytical accuracy of clinical chemistry assays. These can be both endogenous and exogenous and can cause falsely elevated or falsely reduced results that can have serious clinical consequences. The most widely recognized endogenous interference is hemolysis. Hemolysis occurs when red blood cells are ruptured either in vivo, or in vitro, and their contents are released into blood plasma. There can be increased values [potassium, aspartate aminotransferase (AST)] and decreased values (sodium) because of the concentration gradient between the cells and plasma. Hemoglobin and other intracellular components can interfere with chemical reactions, and also hemoglobin absorbs light at 415 nm and, therefore, can cause an apparent increase in concentration for assays using this wavelength of absorbance [1]. Icterus is caused by elevated bilirubin present in the serum resulting in a yellow coloured serum, and is most frequently caused from liver disease. Lipemia is due to the presence of a high concentration of lipids in the blood, which cause light scattering in spectrophotometric assays.
Although most laboratory staff are fully aware of what the measurement of hemolysis, icterus and lipemia (HIL) indices is meant to achieve, there is a bit of a ‘black hole’ in how their instruments have been set up. Most automated clinical chemistry analysers automatically measure the presence of the serum indices of HIL by absorbance alone and report a number with no units to the user. The manufacturer’s engineers are usually the people who set the analysers up but unfortunately there appears to be a variety of ways to do this, and because there is no ‘correct’ way, differences have inadvertently crept in. Manufacturers use different paired wavelengths for the determination of the serum indices, and have different methods of reporting – numerical (e.g. 1, 2, 3) and category/non-numerical (e.g. +++) [2]. Automatic algorithms, which differ between manufacturer, will either allow, or not allow an individual result to be reported.
A typical large laboratory in the UK analyses approximately 6000 specimens for serum indices daily. Although internal quality control (IQC) is carried out on all clinical chemistry ‘real’ assays prior to results being released, it is not routine for laboratories to apply IQC procedures to serum indices. Laboratories accredited to ISO15189:2012 are accredited at the reportable test level, and as serum indices are not reported, these are not always covered within the scope of accreditation. There is, therefore, a large scope for errors within a laboratory’s serum indices to impact on analytical results.
EQA scheme design
Birmingham Quality has established an external quality assessment (EQA) scheme for serum indices – UK NEQAS for Serum Indices. The scheme not only looks at hemolysis, icterus and lipemia as individual analytes but also systematically looks at the impact that these serum indices have on particular analytes, which changes from month to month, and is called ‘Analyte X’. Laboratories are asked whether they would report the result for the measured analyte based on the serum indices. This scheme is available worldwide but the majority of participants are UK based.
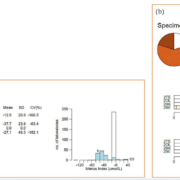
The innovative report style from Birmingham Quality allows two data presentations from the same analyte – standard report format for numerical (index) data, and pie charts for the category data (Fig. 1a and 1b, respectively). Method mean concentrations are used as the target value for numerical results and the consensus category for individual manufacturers.
HIL performance
By their very nature, indices have no real ‘unit’ associated with them. That said, they do have a tenuous linkage with concentrations of hemoglobin, bilirubin and triglyceride. The usual units for these analytes in the UK are g/L, µmol/L and mmol/L and many instruments are indeed set up in these units. Similarly, many are set up in mg/dL, which though perfectly common in the US and the non-UK, non-Scandinavian, European countries, are never used in the UK but yet have representation here. Now although this doesn’t particularly matter to an individual laboratory, we have the unintended situation of two machines in the same laboratory having been set up in different units.
Hemolysis
Semi-quantitative hemolysis results are correlated to an approximate hemoglobin concentration, and, as such, the units are g/L, mg/dL or µmol/L.
Hemolysed specimens are prepared endogenously by allowing serum to remain on red blood cells for a period of time, or by the addition of exogenous material. There is generally good agreement within a method and the between method imprecision [percent coefficient of variation (%CV)] is fairly consistent across the concentration range 0.5–10 g/L at approximately 5 % for Abbott and Roche, and approximately 12 % for Siemens and Ortho J&J.
Icterus
Semi-quantitative icterus results are correlated to an approximate bilirubin concentration, and, as such, the units are µmol/L or mg/dL.
Icteric specimens are prepared as either endogenous, or by the addition of exogenous material. The between method imprecision varies between manufacturer and is likely to reflect the differences in measurement approach. Differences between methods have been observed. For example, an unspiked sample was distributed which had endogenous elevated triglycerides (specimen 111C in Fig. 1). Two methods – Beckman Synchron and Beckman AU Olympus – have identified a significant amount of icterus present, whereas other major methods haven’t. Analytically this is because of secondary interference due to overlapped absorbance not being corrected. Clinically this means that there is the potential that results would not be reported because of an incorrect icterus result being reported on a lipemic sample, when analytically they may be valid for icterus.
Lipemia
Lipemia results are roughly correlated to triglyceride concentration and in most cases are calibrated/anchored to intralipid concentration, and reported as g/L, mg/dL or mmol/L. Many manufacturers use more than one ‘unit’ for serum indices. For hemolysis and icterus the numerical results are obviously different; however, for lipemia there is only a factor of 1.13 between g/L and mmol/L. As serum indices are usually reported without a unit there is the potential for error if the units that are measured are not the same as when interpreted.
Lipemia shows higher between method imprecision %CVs on all methods. Specimens are either distributed with endogenously elevated lipids, or intralipid is added. Both cases show similarly high %CV, which is likely to be due to differences in the light scattering of turbid samples. However, there is also the possibility that although participants are advised to mix EQA material prior to analysis, they may not always. This problem is not unique to EQA specimens, as a delay in analysis of separated clinical specimens, or ‘add-on’ tests means that lipemic material could have separated before being sampled.
Analyte X
Analyte X is a unique and sophisticated concept where the laboratory is challenged for a specified different analyte every month. The participant reports the value obtained for this variable analyte and also an interpretation of whether they would report that result for analyte X based on the serum indices they obtained. This allows an assessment of the impact of serum indices on the numerical result of the analyte, and the participant can directly compare different methods. The interpretative element demonstrates for the first time the variation in clinical practice.
Significant differences in practice for the interpretation of serum indices both within and between manufacturers have been observed. For example, three specimens of the same base material were distributed with varying degrees of hemolysis for the analysis of total protein (Fig. 2). Specimen 106A was slightly hemolysed (overall consensus mean 0.7 g/L) and specimen 106C was grossly hemolysed (overall consensus mean 4.7 g/L). Beckman AU, Roche and Siemens did not show any significant change in the total protein result reported for all three specimens; however, Abbott and Ortho J&J both showed an increase in total protein as the amount of hemolysis increased. All methods showed a mixture of whether a laboratory would or would not report the total protein result, even with the grossly hemolysed specimen (Fig. 2b). This shows differences in algorithms being used even within manufacturers. Approximately 20 % of Abbott participants and approximately 25% of Ortho J&J participants would report an elevated total protein result in the presence of gross hemolysis. The consequences of this erroneously high total protein result could lead to additional testing. This would not only cause the clinician to unnecessarily waste time, but could also cause concern for the patient.
Discussion
With the increase of tracked automation, separated specimens are no longer ‘handled’ by the operator, and, therefore, no visual inspection takes place. The laboratory is entirely reliant on the use of algorithms based on absorbance of the specimen to decide whether individual analytical results should be reported or not. Serum indices are usually only measured once. This may be soon after a specimen has been centrifuged or sometime later. It is known that lipemic specimens ‘separate’ over time; therefore, any delay in analysis, or the addition of any subsequent ‘add-on’ tests, may result in the sampling of an incorrect portion of serum. Serum indices are not usually reported to a clinician, and may be presented on the analyser only as a number with no units. Therefore, there is a reliance by the laboratory on the manufacturer that the numbers correlate with the correct cut-off values for particular analytes. Data from the UK NEQAS for Serum Indices Scheme has shown variation within a manufacturer on the reporting of analytes based on the HIL result; therefore, either laboratories are changing cut-offs, or manufacturers are not setting laboratories up the same way. Manufacturers extensively test their assays for interferences prior to being released, and inform laboratories of this in their kit inserts. The laboratory is responsible for verifying that these levels of interference are suitable for their requirements.
Hemolysed, icteric or lipemic specimens either result in patients not receiving results, and, therefore, needing to be re-bled, or incorrect results being reported for specific analytes. Either way, the patient’s care is affected. Hemolysis is considered one of the most common interferents and the incidence with which a laboratory receives hemolysed specimens varies widely and is dependent on how specimens are collected [2]. A study of the incidence of hemolysed samples in an Emergency Medicine department in the UK concluded that 10.7 % of specimens were hemolysed over the seven-day sampling period. [3] Overall incidence data for specimens/tests rejected because of hemolysis, icterus or lipemia is not available; however, the impact on the laboratory, clinician and patient is likely to be significant.
Conclusion
The UK NEQAS for Serum Indices Scheme has shown that there is generally good analytical performance within individual manufacturers for hemolysis and icterus. Lipemia shows more variation in results. Variations are observed between manufacturers and in the application of the serum index to interpretation of a clinical chemistry result.
Automation has allowed the clinical chemistry analysis a more rapid throughput; however, human contact with specimens has now been reduced, which has increased reliance on computer algorithms. The UK NEQAS for Serum Indices Scheme has, and continues to show, variation in practice which consequently affects patient care both in terms of repeat testing and the validity of results.
References
1. Thomas L. Haemolysis as influence and interference factor. eJIFCC 2002; 13(4): http://www.ifcc.org/media/477061/ejifcc2002vol13no4pp095-098.pdf.
2. Farrell CJL, Carter AC. Serum indices: managing assay interference. Ann Clin Biochem 2016; 53: 527–538.
3. Berg JE, Ahee P, Berg JD. Variation in phlebotomy techniques in emergency medicine and the incidence of haemolysed samples. Ann Clin Biochem 2011; 48: 562–565.
The authors
Rachel Marrington* PhD, FRCPath and Finlay MacKenzie MSc (Director and consultant clinical scientist)
Birmingham Quality (UK NEQAS), Queen Elizabeth Hospital Birmingham, Birmingham, UK
*Corresponding author
E-mail: rachel.marrington@uhb.nhs.uk